



UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C.DC 20549
SCHEDULE 14A
Proxy Statement Pursuant to SectionPROXY STATEMENT PURSUANT TO SECTION 14(a) of the Securities
OF THE SECURITIES EXCHANGE ACT OF 1934
Exchange Act of 1934 (Amendment(Amendment No. )
 |  | Filed by the Registrant |   | Filed by a Party other than the Registrant |
| Check the appropriate box: | |
  | Preliminary Proxy Statement |
  | Confidential, for |
  | Definitive Proxy Statement |
  | Definitive Additional Materials |
  | Soliciting Material |

Moderna, Inc.
(Name of Registrant as Specified Inin Its Charter)
(Name of Person(s) Filing Proxy Statement, if other than the Registrant)Registrant)
| Payment of Filing Fee (Check | ||
  | No fee required. | |
  | Fee paid previously with preliminary materials. | |
 | Fee computed on table | |
 | ||
 | ||



MONDAY, MAY 6, 2024 8:00 a.m., Eastern Time www.virtualshareholdermeeting.com/MRNA2024 |
HOW TO VOTE Review your proxy statement and vote in one of three ways: | |
 | Internet www.proxyvote.com |
 |
1-800-690-6903 |

Notice of Annual Meeting of Stockholders
|
|
  |
| |
 |
| |
 |
| |
YOUR VOTE IS IMPORTANT.IMPORTANT. Even if you plan to participate in the Annual Meeting, we urge you to submit your proxy in advance to ensure your shares are represented. This will not affect your right to participate in the meeting and to vote your shares at that time. For additional information on voting and participating in the meeting, please see “Information About the 20212024 Annual Meeting of Stockholders”Shareholders” beginning on page 58.83.
To the StockholdersShareholders of Moderna, Inc.:
You are cordially invited to the Annual Meeting of StockholdersShareholders of Moderna, Inc., which will be held on Wednesday, April 28, 2021,Monday, May 6, 2024, beginning at 8:00 a.m., Eastern Time (the Annual Meeting), for the following purposes:
To elect three Class III directors, each to serve for a three-year term expiring at the 2024 annual meeting of stockholders;
To approve, on a non-binding, advisory basis, the compensation of our named executive officers;
To ratify the appointment of Ernst & Young LLP as our independent registered public accounting firm for the year ending December 31, 2021; and
To transact such other business as may be properly brought before the Annual Meeting or any adjournment or postponement thereof.
| 1. | To elect three Class III directors, each to serve for a three-year term expiring at the 2027 annual meeting of shareholders; |
| 2. | To approve, on a non-binding, advisory basis, the compensation of our named executive officers; |
| 3. | To ratify the appointment of Ernst & Young LLP as our independent registered public accounting firm for the year ending December 31, 2024; |
| 4. | To approve a management proposal to amend our Certificate of Incorporation to provide shareholders the right to call a special meeting; |
| 5. | To approve a management proposal to amend our Certificate of Incorporation to reflect new Delaware law provisions allowing for officer exculpation; and |
| 6. | To transact such other business as may be properly brought before the Annual Meeting or any adjournment or postponement thereof. |
The Annual Meeting will be conducted virtually due to the COVID-19 pandemic and related public health concerns.virtually. You will be able to participate in the Annual Meeting online and submit your questions during the meeting by visiting www.virtualshareholdermeeting.com/MRNA2021.MRNA2024. You also will be able to vote your shares electronically during the Annual Meeting. For more information about our virtual Annual Meeting, please see “Information About the 20212024 Annual Meeting of Stockholders”Shareholders” beginning on page 58.83.
Our Board of Directors has fixed the close of business on March 1, 2021,7, 2024, as the Record Date for determining the stockholdersshareholders that are entitled to notice of and to vote at the Annual Meeting and any adjournment or postponement thereof.
This proxy statement and our Annual Report on Form 10-K for the year ended December 31, 2020,2023, are first being mailed on or about March 11, 2021,[ ], 2024, to all stockholdersshareholders entitled to vote at the Annual Meeting. These materials also are available at www.proxyvote.com, using the control number provided with your materials.
By order of the Board of Directors,
Stéphane Bancel
ChiefExecutiveOfficerandDirector
Cambridge, Massachusetts
March 10, 2021

moderna 2021 Proxy statement |1Stéphane Bancel
Chief Executive Officer and Director
Cambridge, Massachusetts
March [ ], 2024
 | 2024 Proxy statement | 1 |
This summary highlights certain information from this Proxy Statement, but does not contain all the information that you should consider. Please read the entire Proxy Statement before voting your shares. For more complete information regarding Moderna’s 20202023 performance, please review our Annual Report on Form 10-K for the year ended December 31, 2020.2023, including the sections captioned "Special Note Regarding Forward-Looking Statements" and "Risk Factors", for a description of the substantial risks and uncertainties related to the forward-looking statements included herein. Expected product launches are subject to, among other risks, assumptions and uncertainties, clinical trial, regulatory and commercial success, and availability of supply.
When |
Where |
Record date |
| The meeting will be held virtually at | March |
The matters we will act upon at the Annual Meeting are:
Proposal | Board voting
| Where to find more information | |||
Proposal 1: Elect three Class III directors, each for a three-year term |  |
| FOR all nominees | Page | |
Proposal 2: Approve, on a non-binding, advisory basis the compensation of our named executive officers |  |
 | FOR | Page | |
Proposal 3: Ratify the appointment of Ernst & Young LLP as our independent registered public accounting firm for |  |
 | FOR | Page | |
| Proposal 4: Amend our Certificate of Incorporation to provide shareholders the right to call a special meeting |  | FOR | Page 79 | ||
| Proposal 5: Amend our Certificate of Incorporation to reflect new Delaware law provisions allowing for officer exculpation |  | FOR | Page 81 |
moderna 2021 Proxy statement |2
 | 2024 Proxy statement | 2 |
Name | Age | Independent | Principal occupation | Committees | Other public boards | Director since |
Class III directors nominated for re-election for a three-year term | ||||||
Robert Langer, Sc.D. | 72 |  | Academic Co-Founder, Moderna; | N&CG | 4 | 2010 |
Elizabeth Nabel, M.D. | 69 |  | EVP for Strategy, ModeX Therapeutics and Former President, Brigham Health | PD, N&CG | 1 | 2015* |
Elizabeth Tallett | 71 |  | Former Principal, Hunter Partners | Audit (chair), C&T | 4** | 2020 |
Continuing directors | ||||||
Noubar B. Afeyan, Ph.D, Chairman of the Board | 58 |  | Co-founder and Chairman, Moderna; CEO, Flagship Pioneering | N&CG (chair) | 1 | 2010 Chairman since 2012 |
Stéphane Bancel | 48 |
| Chief Executive Officer, Moderna |
| 1 | 2011 |
Stephen Berenson | 60 |  | Managing Partner, Flagship Pioneering | Audit C&T (chair) | 1 | 2017 |
Sandra Horning, M.D. | 72 |  | Co-founder and Advisor, EQRx | PD (chair) | 2 | 2020 |
François Nader, M.D. | 64 |  | Former President, CEO and Executive Director, NPS Pharmaceuticals | C&T | 2 | 2019 |
Paul Sagan | 62 |  | Senior Advisor and Executive-in-Residence, | Audit C&T N&CG | 1 | 2018 |
* Dr. Nabel was a director from December 2015 to July 2020, when she resigned from the Board after the NIH identified Brigham and Women’s Hospital as one of 99 sites to be involved in the Phase 3 clinical trials of the Moderna vaccine to alleviate any potential concern about the conduct or outcome of the vaccine trials. Dr. Nabel was reappointed to the Board on March 10, 2021, after she stepped down from Brigham Health. ** Ms. Tallett has confirmed that she will be retiring from one of her public company boards in 2021, and will only serve on 4 total public boards (including Moderna) by the end of May 2021. | ||||||
moderna 2021 Proxy statement |3

|
| To deliver the greatest possible impact to people through mRNA medicines | 
|
Moderna is a leader in the creation of the field of mRNA medicines. Through the advancement of mRNA technology, Moderna is reimagining how medicines are made and transforming how we treat and prevent disease for everyone. By working at the intersection of science, technology and health for more than a decade, the Company has developed medicines at unprecedented speed and efficiency, including one of the earliest and most effective COVID-19 vaccines.
Moderna’s mRNA platform has enabled the development of therapeutics and vaccines for infectious diseases, immuno-oncology, rare diseases and autoimmune diseases. With a unique culture and a global team driven by the Moderna values and mindsets to responsibly change the future of human health, Moderna strives to deliver the greatest possible impact to people through mRNA medicines.
$11.7 billion
|
|
| |||
| 48% U.S. market share |
|
programs | ||
| |||||
| in net product sales from COVID-19 vaccines in 2023. | for COVID-19 vaccine sales to the | including Phase 3 programs for | |||
| $13.3billion | 45 programs | 19 markets | |||
| in cash, cash equivalents and investments as of December 31, | in development, reflecting continued investment in the pipeline, laying the groundwork for future growth and profitability. | with Moderna employees around the globe as of December 31, |
Commercial Execution:We are focused on commercial execution to drive sales growth and profitability. This includes building on the current momentum from U.S. Spikevax sales and preparing for multiple product launches, including the expected launch of our RSV vaccine candidate and other potential launches in 2025 and beyond.
Disciplined Investment:We plan to continue to review our cost structure to find efficiencies, and to remain disciplined around spending. We expect to leverage our existing manufacturing and commercial infrastructure as we launch new products in our respiratory franchise in 2024 and 2025.
Executing on Late-Stage Pipeline:We are focused on advancing our late-stage pipeline to drive organic sales growth. Moderna expects to launch up to 15 products in the next five years, with up to four of those launches possibly occurring by 2025.
 | 2024 Proxy statement | 3 |
At the Annual Meeting, three Class III directors will be elected for a three-year term. The Class III directors up for election are set forth below.
| Name | Age | Independent | Principal occupation | Committees* | Other public boards | Director since | ||||
| Class III directors nominated for re-election for a three-year term | Audit | Comp | Nom Gov | Prod Dev | Sci Tech | |||||
| Robert Langer, Sc.D. | 75 |  | David H. Koch Institute Professor, MIT; Academic Co-Founder, Moderna |  |  | 2 | 2010 | |||
| Elizabeth Nabel, M.D. | 72 |  | Former President, Brigham Health |  |  |  | 3 | 2015** | ||
| Elizabeth Tallett | 74 |  | Former Principal, Hunter Partners |  |  | 2 | 2020 | |||
| Continuing directors | ||||||||||
| Noubar Afeyan, Ph.D. Chairman | 61 |  | CEO, Flagship Pioneering; Co-founder and Chairman, Moderna |  | 0 | 2010 Chairman since 2012 | ||||
| Stéphane Bancel | 51 | Chief Executive Officer, Moderna | 0 | 2011 | ||||||
| Stephen Berenson | 63 |  | Managing Partner, Flagship Pioneering |  |  | 1 | 2017 | |||
| Sandra Horning, M.D. | 75 |  | Former Chief Medical Officer and Global Head of Product Development, Roche |  |  |  | 3 | 2020 | ||
| François Nader, M.D. | 67 |  | Former President, CEO and Executive Director, NPS Pharmaceuticals |  |  |  | 1 | 2019 | ||
| Paul Sagan | 65 |  | Catalyst Advisor, General Catalyst |  |  | 0 | 2018 | |||
Chairman |
Member | * Comp = Compensation and Talent Nom Gov = Nominating and Corporate Governance Prod Dev = Product Development Sci Tech = Science and Technology | ** Dr. Nabel was a member of our Board from December 2015 to July 2020, and rejoined the Board in March 2021, following her retirement from Brigham Health. |
| The Board of Directors recommends a vote “FOR” the election of each of the three nominees as a Class III director to serve for a three-year term. For more information on the nominees, see page 8. |
 | 2024 Proxy statement | 4 |

In 2023, our Board of Directors contributed to Moderna’s strategic advancement through its oversight of management’s execution of our business plans and strategy. Advice and guidance from the full Board, and relevant Committees where applicable, was instrumental in the following key accomplishments, among others, in 2023.
| Commercial Execution | 2023 marked our first year of | |
| Pipeline Advancement | The Board and Product Development Committee oversaw investments to advance our pipeline, such that by year-end 2023, nine of our 45 programs were late-stage, including seven programs in Phase 3 and two rare disease programs. This significant advancement of our programs lays the foundation for future growth. | |
| Capital Allocation | The Board continued to oversee significant capital allocation decisions in 2023. This included investments in research & development to advance our pipeline, both internally and through external collaborations. It also included investments in our internal manufacturing capabilities in Norwood and Marlborough, the UK, Australia, and Canada, and the decision to right-size our manufacturing footprint and to restructure our relationships with contract manufacturers as we |
|
| |
|
| The Board and Nominating and Corporate Governance Committee are continuing to advance our Board succession planning and recruitment of new directors |
|
| In response to investor feedback, our Board approved several enhancements to our governance practices, including (i) the adoption of majority voting for |
| Human Capital and ESG Efforts | The Board and its committees oversaw human capital and environmental, social and governance (ESG) |
 | 2024 Proxy statement | 5 |
Shareholders will be asked to approve, on a non-binding, advisory basis, the compensation of Moderna’s named executive officers. This is commonly known as a “say on pay” proposal.
Over the last several years, we have evolved our executive compensation programs in order to:
| • | Enhance alignment with shareholders through our pay-for-performance philosophy; |
| • | Align with competitive market practices; and |
| • | Reflect input received from shareholders. |
| Overall Performance | •We made significant progress in 2023 advancing our mRNA platform and our broad clinical pipeline. •We achieved 48% U.S. retail market share for the 2023 fall season, an 11 percentage point increase from 2022. •Corporate performance did not meet expectations for our financial goals, falling short on product sales and operating income objectives. | |
|
| •Modest increases in 2023, reflective of merit and market adjustments to |
|
| •2023 corporate performance did not meet our expectations, resulting in our Executive Committee |
moderna 2021 Proxy statement |4
|
| |
| Long-Term Incentive | •Increased the | |
|
| |
|
| |
|
•92% at-risk target compensation for CEO, and •CEO realizable pay demonstrates a strong correlation between stock price performance and pay; see page 41 for more details in the “Pay for Performance” section of the | |
| Transparency | •Augmented our CD&A to provide shareholders with visibility on the goals underlying our short-term and long-term incentive programs. | |
Our executive compensation program is based on a pay-for-performance philosophy, which is reflected in both our annual and long-term incentive compensation programs. We believe that a significant portion of each executive’s compensation should be variable and at-risk and tied to the achievement of pre-established Company performance goals that drive value creation for our business and align our executives’ interests with those of our shareholders. The largest component of our 2023 long-term incentive compensation program is delivered in the form of stock options, which directly aligns payouts with outcomes for our shareholders.
The charts below set forth the target total compensation mix for Mr. Bancel, our Chief Executive Officer, and our average NEO’s target compensation for 2023.
| CEO Target Pay Mix | Average NEO Target Pay Mix | |
 |  |
 | The Board of Directors recommends a vote “FOR” approval, on a non-binding, advisory basis, of the compensation |
 | 2024 Proxy statement | 6 |
| • | We are asking shareholders to ratify the appointment of Ernst & Young LLP as our independent auditor for the year ended December 31, 2024. |
| • | The Audit Committee is committed to ensuring the independence of Ernst & Young, and has taken steps in recent years to ensure that we minimize spending on items with the firm other than the audit or audit-related matters. |
moderna 2021 Proxy statement |5
 | The Board of Directors recommends a vote “FOR” the ratification of the appointment of Ernst & Young LLP as our independent registered public accounting firm for the year ending December 31, 2024. For more information, see page 76. |
| • | In response to feedback from our investors, our Board of Directors is proposing to amend our Certificate of Incorporation to permit shareholders representing at least 20% of our outstanding shares to call a special meeting of shareholders. |
| • | We believe that granting shareholders the right to call a special meeting, while setting a threshold of 20%, will grant investors with a sufficiently large economic and voting interest a valuable right, while also protecting against the potential disruption and possible loss to long-term shareholder value posed by setting a lower threshold. |
| • | The special meeting right builds on other efforts earlier in 2024—including the adoption of a proxy access bylaw and the adoption of majority voting for uncontested director elections—to respond to investor feedback regarding Moderna’s governance practices. |
 | The Board of Directors recommends a vote “FOR” the proposal to amend our Certificate of Incorporation to provide shareholders the right to call a special meeting. For more information, see page 79. |
| • | In August 2022, Delaware law was amended to permit Delaware companies, like Moderna, to limit the personal liability of certain officers in limited circumstances, similar to protection that was already afforded to directors. |
| • | The amended law permits a company to exculpate these officers for direct claims brought by shareholders for breach of an officer’s duty of care, but would not eliminate an officer’s monetary liability for breach of fiduciary duty claims brought by the company or in connection with derivative claims brought by shareholders on behalf of the company. It also would not allow officers to be exculpated for breaches of the duty of loyalty to the company, or acts or omissions that are not in good faith or that involve intentional misconduct, a knowing violation of law, or a situation in which an officer derived an improper personal benefit from a transaction. |
| • | The Board of Directors is proposing to amend our Certificate of Incorporation to allow for the exculpation of officers of Moderna, consistent with the recent changes to Delaware law and to provide our executives similar protections to those already extended to our directors. |
| • | The Board of Directors recognizes that our officers are called upon to make critical decisions, and that in an increasingly litigious environment our executive team can be exposed to substantial personal liability from litigants seeking to impose liability on the basis of hindsight and regardless of merit. |
| • | The Board of Directors believes that providing the protection permitted under Delaware law will provide comfort to and empower our officers to best exercise their business judgment in the interests of Moderna and its shareholders, which will encourage agility and critical decision-making, while also enabling us to continue to attract and retain experienced and high-quality officers to the Company. |
 | The Board of Directors recommends a vote “FOR” the proposal to amend our Certificate of Incorporation to allow for officer exculpation. For more information, see page 81. |
 | 2024 Proxy statement | 7 |
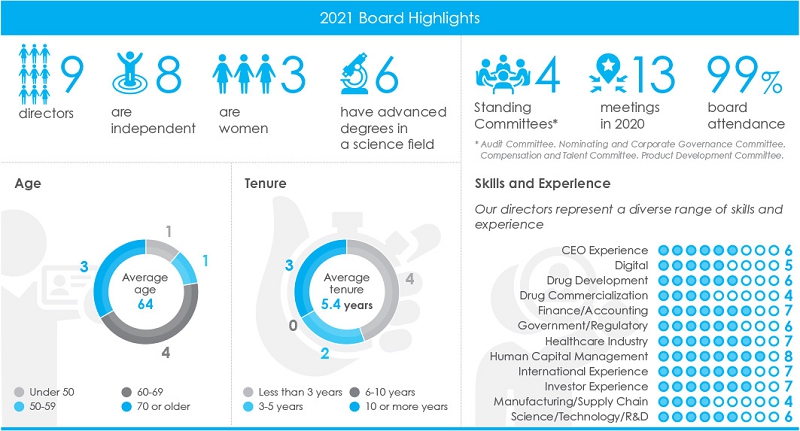
Our Board of Directors currently has nine members, who are divided into three equal classes with staggered three-year terms. At the Annual Meeting, three Class III directors will be elected for a three-year term. Each of these nominees is a Class III director whose current term is expiring. Each director will continue in office until the election and qualification of a successor or until such director’s earlier death, resignation, or removal.
Our Nominating and Corporate Governance Committee has recommended, and our Board of Directors has approved, Robert Langer, Sc.D., Elizabeth Nabel, M.D. and Elizabeth Tallett as nominees for election as Class III directors at the Annual Meeting.
Dr. Langer has served on Moderna’sthe Board since 2010, Dr. Nabel has served on the Board from Decembersince 2015, to July 2020, and was reappointed in March 2021, and Ms. Tallett joinedhas served on the Board in Julysince 2020. Ms. Tallett was originally recommended for the Board by a third-party search firm.
If you are a stockholdershareholder of record and you sign your proxy card or vote over the Internet or by telephone but do not give instructions with respect to the voting of directors, your shares will be voted FOR the election of Dr. Langer, Dr. Nabel and Ms. Tallett. We expect that the nominees will serve if elected. However, if a director nominee is unable or declines to serve as a director at the time of the Annual Meeting, proxies will be voted for any nominee who is designated by our Board of Directors to fill the resulting vacancy. If you own your Moderna stock through a broker, bank, or other nominee and you do not give voting instructions, then your shares will not be voted on this matter. For more information, please see “Information About the 20212024 Annual Meeting of Stockholders—Shareholders—What if I do not specify how my shares are to be voted?” on page 60.85.
The election of the Class III directors requires a plurality votemajority of the shares of our common stock present in person or by proxy at the Annual Meeting and entitled to vote thereonvotes properly cast to be approved. If a director nominee does not receive a majority of the votes cast in favor of his or her election to the Board, the director nominee will be required to tender his or her resignation to the Board for consideration and action by the Board.
  | The Board of Directors recommends a vote “FOR” the election of each of the three nominees as a Class III |
moderna 2021 Proxy statement |6
 | 2024 Proxy statement | 8 |

Age:
Director since: 2010
Committees: •Nominating and Corporate Governance
| Robert Langer, Sc.D. | |
| ||
Dr. Langer is | ||
Other Public Boards |
| |
•
Seer, Inc. (since 2020) • Puretech Health plc (since 2015) • Abpro Korea • •Rubius Therapeutics, Inc. (2015-2019) • Kala Pharmaceuticals, Inc. (2009-2018) | Education • B.S. in chemical engineering from Cornell University • Sc.D. in chemical engineering from the Massachusetts Institute of Technology | |
Dr. Langer has been an Institute Professor at the Massachusetts Institute of Technology (MIT) since 2005, and He is an elected member of the National Academy of Sciences, the National Academy of Engineering, the National Academy of Medicine and the National Academy of Inventors. Dr. Langer has written over 1,550 articles and also has 1,450 issued or pending patents worldwide. Dr. Langer’s patents have been licensed or sublicensed to over 400 pharmaceutical, chemical, biotechnology and medical device companies. | ||

Age:
Director since:
Independent
Committees: •
• Product Development
2023 Attendance:100% | Elizabeth Nabel, M.D. | |
| ||
Dr. Nabel | ||
Other Public Boards |
| |
• Medtronic plc (since 2014) •Lyell Immunopharma, Inc. (since 2021) •Accolade, Inc. (since 2021) | Education • B.A. from St. Olaf College • M.D. from Cornell University Medical College • Postgraduate training in internal medicine and cardiovascular diseases at Brigham and Women’s Hospital and Harvard University | |
From 2010 to
| ||
 | 2024 Proxy statement | 9 |
moderna 2021 Proxy statement |7
Age:
Director since: 2020
Independent
Committees: Audit (Chair) Compensation and Talent
| Elizabeth Tallett | |
| ||
Ms. Tallett | ||
Other Public Boards |
| |
• Elevance Health, Inc. (previously Anthem, Inc.) (since 2013), Chair since 2018 • •Principal Financial Group • Meredith Corp., Inc. Education •
| Nottingham University with a dual first class honours degree in mathematics and economics | |
Ms. Tallett has spent more than 35 years in strategic leadership and operational roles in worldwide biopharmaceutical and consumer products | ||

Age:
Director since: 2010
Chairman since: 2012
Term expires:
Independent
Committees: • Nominating and Corporate
(Chair)
| Noubar | |
| ||
Dr. | ||
Other Public Boards |
| |
• Rubius Therapeutics, Inc. • Seres Therapeutics, Inc. (2012-2020) • Evelo Biosciences, Inc. (2014-2019) • Kaleido Biosciences, Inc. (2015-2019) Education •
| B.S. in chemical engineering from McGill University • Ph.D. in biochemical engineering from the Massachusetts Institute of Technology | |
Dr. Afeyan founded Flagship Pioneering, a company established in 1999 that creates bioplatform companies to transform human health and sustainability, and serves as its Senior Managing Partner and Chief Executive Officer. He | ||
 | 2024 Proxy statement | 10 |
moderna 2021 Proxy statement |8
Age:
Director since: 2011
Term expires:
2023 Attendance: 100% | Stéphane Bancel | |
| ||
As our CEO for more than a decade, Mr. Bancel | ||
Other Public Boards |
| |
• Qiagen N.V. • Syros Pharmaceuticals, Inc. (2013-2017) | Education • Master of Engineering degree from École Centrale Paris • Master of Science in chemical engineering from the University of Minnesota • M.B.A. from Harvard Business School | |
Mr. Bancel has served as our | ||

Age:
Director since: 2017
Term expires:
Independent
Committees: •
100% | Stephen Berenson | |
| ||
Mr. Berenson | ||
Other Public Boards |
| |
• Seres Therapeutics, Inc. (since 2019), Chair | Education • S.B. in mathematics from the Massachusetts Institute of Technology | |
Mr. Berenson is a Managing Partner at Flagship Pioneering. He oversees Flagship’s capital formation and business development activities, is deeply involved in firm-wide strategy and the firm’s talent agenda and is a member of the firm’s resource allocation committee. Prior to | ||
 | 2024 Proxy statement | 11 |
Age:
Director since: 2020
Term
Independent
Committees: • Product Development (Chair) •Nominating and Corporate Governance •Science and Technology
| Sandra Horning, M.D. | |
| ||
Dr. Horning | ||
Other Public Boards |
| |
• Gilead Sciences, Inc. (since 2020) • Olema Pharmaceuticals, Inc. (since 2020) •EQRx, Inc. (2021-2023) | Education • M.D. from the University of Iowa School of Medicine • Completed internal medicine training at the University of Rochester • Post-graduate fellowship in Oncology and Cancer Biology at Stanford University | |
Dr. Horning was the Chief Medical Officer and Global Head of Product Development of Roche, Inc., from 2014 until her retirement in 2019, and, | ||
moderna 2021 Proxy statement |9

Age:
Director since: 2019
Term expires:
Independent
Committees: • Compensation and Talent (Chair) • Product Development •Science and Technology
100% | François Nader, M.D. | |
| ||
Dr. Nader | ||
Other Public Boards |
| |
• •Acceleron Pharma Inc. • Alexion Pharmaceuticals, Inc. • Prevail Therapeutics Inc. (2018-2021), Chair • Clementia Pharmaceuticals Inc. (2014-2019) • Advanced Accelerator Applications S.A. (2016-2018) • Baxalta •
NPS Pharmaceuticals (2008-2015) | Education • French doctorate in medicine from St. Joseph University in Lebanon • Physician Executive M.B.A. from the University of Tennessee | |
Dr. Nader served as President, | ||
 | 2024 Proxy statement | 12 |
Age:
Director
Term
Independent
Committees: •
•
Nominating
100% | Paul Sagan | |
| ||
Mr. Sagan | ||
Other Public Boards |
| |
• VMware, Inc. • Akamai Technologies, Inc. (2005-2019) • EMC Corp. (2007-2016) • iRobot, Inc. (2010-2015) Education •
| B.S. from the Medill School of Journalism at Northwestern University | |
Mr. Sagan is a | ||
 | 2024 Proxy statement | 13 |
moderna 2021 Proxy statement |10
Composition of our Board of Directors
Our Board currently consists of nine members, but the Board has the authority to increase or decrease that size depending on an assessment of its needs and other relevant circumstances at any given time.
Our Nominating and Corporate Governance Committee (Nominating Committee) and our Board of Directors consider a broad range of factors when selecting nominees. We strive to identify candidates who will further the interests of our stockholders.shareholders. Among other things, we expect that all of our directors will have the following experience and traits:
an established record of professional accomplishment at a strategic or policymaking level in a business, government, non-profit, or academic organization of high standing;
| • | substantial experience at a strategic or policymaking level in a business, government, non-profit, or academic organization of high standing, able to contribute to Moderna’s strategic growth and able to offer advice and guidance to Moderna’s senior management based on that experience; |
| • | highly accomplished in their respective fields; |
| • | the ability to contribute positively to the Board’s collaborative culture; |
| • | knowledge of our business; |
| • | understanding of the competitive landscape facing our business; and |
| • | expertise relevant to our growth and business strategy. |
the ability to contribute positively to the board’s collaborative culture;
knowledge of our business;
understanding of the competitive landscape facing our business; and
expertise relevant to our growth and business strategy.
In addition, every nominee must have sufficient time and availability to devote to Moderna’s affairs, a reputation for high ethical and moral standards, an understanding of the fiduciary responsibilities assumed by public company directors, and a demonstrated commitmentthe time and energy necessary to diligently carry out those responsibilities, and role model our values of quality, integrity and respect.demonstrate a willingness to embrace the Moderna Mindsets, further described in “ESG Topics—Human Capital Management” on page 31.
The Nominating Committee reviews Board succession regularly, and in considering director candidates is focused on those individuals who can help guide Moderna as it executes on its strategy over the next several years. This includes seeking out candidates with experience overseeing global pharmaceutical companies, individuals with significant governmental or public policy experience, and individuals who can help guide our growth as a digitally enabled and highly innovative company.
In building our Board, we also believe that the following skills and experiences, while not exhaustive, are helpful in ensuring that our directors collectively possess the skills and backgrounds necessary for us to execute on our strategic plans and to exercise the Board’s oversight responsibilities on behalf of our shareholders. Skills and experiences shown below are generally reflective of the individual having worked in the area, rather than experience obtained as a director in the relevant field.
Skill/Experience | Afeyan | Bancel | Berenson | Horning | Langer | Nabel | Nader | Sagan | Tallett |
CEO Experience |  |  |  |  |  |  | |||
|  |  | ✓ |  |  |  | |||
Drug Development |  |  |  |  |  |  | |||
Drug Commercialization | ✓ |  |  |  |  | ||||
Finance/Accounting |  |  |  | ✓ |  |  |  |  | |
Government/Regulatory |  |  |  |  |  |  | |||
Healthcare Industry |  |  |  |  |  |  |  | ||
Human Capital Management |  |  |  |  |  |  |  |  | |
International Experience |  |  |  |  |  |  |  | ||
Investor Experience |  |  |  |  |  |  |  | ||
Manufacturing/Supply Chain |  |  |  |  | |||||
Science/ |  |  | ✓ | ✓ | ✓ | ✓ |
 |  |  |
Our directors hold office until their successors have been elected and qualified or until their earlier death, resignation, or removal. Directors may be removed only for cause by the affirmative vote of the holders of at least two-thirds of the votes that all our stockholdersshareholders would be entitled to cast in an annual election of directors. Any vacancy on our Board of Directors, including a vacancy resulting from an enlargement of the Board, may be filled only by vote of a majority of the directors then in office.
The following Board Diversity Matrix presents our Board diversity statistics in accordance with Nasdaq Rule 5606, as self-disclosed by our current directors. While the Board satisfies the minimum objectives of Nasdaq Rule 5605(f)(3) by having at least one director who identifies as female and at least one director who identifies as a member of an Underrepresented Minority (as defined by Nasdaq Rules), we note that one of our directors also identifies as Middle Eastern. As we pursue future Board recruitment efforts, our Nominating Committee will continue to seek out candidates who can contribute to the diversity of views and perspectives of the Board in accordance with the committee’s Policies and Procedures for Director Candidates. This includes seeking out individuals of diverse ethnicities, a balance in terms of gender, and individuals with diverse perspectives informed by other personal and professional experiences.
Board Diversity Matrix as of March 7, 2024
| Part I: Gender Identity | Female | Male | Non-Binary | Decline to Disclose |
| Directors (9 total) | 3 | 5 | - | 1 |
| Part II: Demographic Background | Female | Male | Non-Binary | Decline to Disclose |
| African American or Black | - | - | - | - |
| Alaskan Native or Native American | - | - | - | - |
| Asian | - | 1 | - | - |
| Hispanic or Latinx | - | - | - | - |
| Native Hawaiian or Pacific Islander | - | - | - | - |
| White | 3 | 5 | - | - |
| Two or More Races or Ethnicities | - | 2 | - | - |
| LGBTQ+ | - | |||
| Did Not Disclose Demographic Background | 1 | |||
| Directors who identify as Middle Eastern | - | 1 | - | - |
OurIn consultation with our Nominating Committee, our Board of Directors ishas determined that a staggered board structure, with directors divided into three classes with staggered terms.terms, remains appropriate for us at this time. At each annual meeting of stockholders,shareholders, one class of directors will be elected for a three-year term to succeed the directors of the same class whose terms are then expiring. The division
A staggered board provides for stability, continuity and experience among our Board. This structure helps to resist pressure to focus on short-term results at the expense of our long-term value and success, which is especially important in our industry given the multi-year time horizons required for the successful development of pharmaceutical products and product candidates. Our Board of Directors into three classes with staggered terms may delay or prevent stockholderalso believes that this structure helps to preserve institutional knowledge that can help us identify new opportunities, such as when our Board noted parallels early in 2020 between our earlier efforts to effectdevelop a changevaccine against Middle Eastern Respiratory Syndrome (MERS) and the disease that came to be known as COVID-19. This led to the successful development and launch of our management or a change in control.first product, our COVID-19 vaccine.
 | 2024 Proxy statement | 15 |
moderna
During 2023, the Moderna Board was focused on continuing to execute on the shift to an endemic, commercial market for the Company’s COVID-19 vaccine, while also advancing the Company’s pipeline of products beyond COVID-19 and right-sizing the Company’s manufacturing footprint to lay the foundation for future growth and profitability.
COVID-19 Vaccine Commercial Launch. In 2023, the market for COVID-19 vaccines further evolved to reflect seasonal market dynamics, with sales shifting significantly to the second half of the year and significantly lower demand than in 2021 Proxy statement |11
Backand 2022. Additionally, 2023 marked the shift to Contents
Stockholder Recommendations for Director Nominations
The Nominating and Corporate Governance Committee welcomes recommendations from stockholders for director nominees. These nominees will be evaluatedan endemic, commercial market in the same manner as nomineesU.S., with most sales for COVID-19 vaccines occurring in the private retail channel. The Board remained focused on strategic oversight of these evolving market dynamics and ensuring that come to the committee’s attention from other sources.
To suggestModerna was ready for a director nominee, a stockholder should send the following information to 200 Technology Square, Cambridge, Massachusetts 02139, (617) 714-6500, Attention: Corporate Secretary:
the name, age, business address and residence addresssignificant product launch of the nominee,
updated version of its COVID-19 vaccine, Spikevax, in the principal occupation or employmentthird quarter of 2023, which targeted the nominee,
the class and numberXBB.1.5 variant of shares that are held of record or are beneficially owned by the nominee, including any derivative positions held or beneficially held,
whether and the extent to which any hedging or other transaction or series of transactions has been entered into by or on behalf of the nomineeSARS-CoV-2. Launching in a commercial market significantly changed dynamics with respect to marketing, contracting, distribution and accounting, all of which called on the Board’s expertise. In the U.S. market, the Moderna team expanded its market share to 48% of the retail market for the 2023 fall vaccination season, up 11 percentage points from 37% in 2022 (Source: IQVIA). The Company expects to leverage its experience in the commercial market for COVID-19 vaccines in the anticipated launch of its RSV vaccine in 2024.
Right-Sizing Our Manufacturing Footprint. Over the course of 2023, evolving market dynamics made clear that the manufacturing footprint that was necessary for the production of our COVID-19 vaccines in a pandemic setting would no longer be necessary following the shift to an endemic market. With the Board’s support and oversight, management acted quickly in the third quarter to right-size the Company’s manufacturing footprint to reflect updated market forecasts. These actions are expected to help return the COVID-19 vaccine franchise to profitability in 2024.
By the end of 2023, Moderna had nine late-stage programs, including seven programs in Phase 3 development and two rare disease programs poised to enter pivotal trials in 2024. The Board and its Product Development Committee played a key role in overseeing the advancement and strategic investment in these programs, laying the groundwork to diversify our business beyond COVID-19 vaccines.
Respiratory Vaccine Franchise. Following the announcement of positive efficacy data for the Company’s vaccine candidate against respiratory syncytial virus (RSV) (mRNA-1345) in older adults in January 2023, the Company moved swiftly to apply for approval from regulators in key markets globally. The Company is anticipating approval and is preparing for commercial launch of this product in 2024. During 2023, the Company advanced Phase 3 studies for three additional respiratory vaccines programs beyond our original COVID-19 and RSV vaccines: seasonal flu (mRNA-1010), next-generation COVID-19, which is designed to be refrigerator-stable (mRNA-1283), and our combination vaccine against seasonal flu and COVID-19 (mRNA-1083). We believe that combination respiratory vaccines have the potential to improve coverage while also reducing disease burden and producing savings for healthcare systems, both through lower cost of administration and lower healthcare costs by preventing or reducing the need for care.
Individualized Neoantigen Therapy (INT). The Board has also been actively engaged in overseeing the advancement of clinical trials and investments to prepare for commercial readiness for our oncology program, INT (previously referred to as our personalized cancer vaccine) (mRNA-4157), which we are advancing in collaboration with Merck. The Phase 3 trial of mRNA-4157 for melanoma launched in 2023 and is enrolling globally. In December 2023, we launched a second Phase 3 trial of mRNA-4157 in non-small cell lung cancer, and this study is also enrolling globally. Moderna broke ground on an additional manufacturing site in Marlborough, Massachusetts in 2023, and this facility is expected to be dedicated to the production of INT.
Latent and Rare Diseases. The Board and its committees continue to oversee our strategy to advance our broader pipeline, including investments in our development programs and the talent to move them forward. This includes advancing our vaccines against latent and rare diseases and in other areas. Our Phase 3 study for our vaccine candidate against cytomegalovirus (CMV) (mRNA-1647), the leading infectious cause of birth defects in the U.S., is fully enrolled and could see an efficacy data readout in 2024. In rare diseases, the Paramount study of our propionic acidemia (PA) program (mRNA-3927) and the Landmark study of our methylmalonic acidemia (MMA) program (mRNA-3705) are both ongoing, and are expected to enter into pivotal studies in 2024.
 | 2024 Proxy statement | 16 |
Governance Updates. In 2023, the Board and Nominating Committee continued to assess feedback from Moderna’s investors regarding its governance practices. As a result of this feedback, early in 2024, the Board approved updates to the Company’s bylaws to adopt majority voting for uncontested director elections and to implement a proxy access bylaw on market terms, which will allow shareholders with a significant, long-term ownership interest to nominate director candidates for inclusion on the annual meeting ballot. The Board also is requesting approval from shareholders to amend the Company’s Certificate of Incorporation to allow shareholders to call a special meeting (see “Proposal 4: Amend Our Certificate of Incorporation to Provide Shareholders the Right to Call a Special Meeting,” beginning on page 79). Additionally, during 2023, our Nominating Committee approved updates to our Corporate Governance Guidelines to require that directors obtain the approval of the Chair of the Committee before taking on any Moderna securities,new board or director roles, as described below under “Director Independence.” Additionally, the Nominating Committee has implemented enhanced screening of potential conflicts as the scope of our business has expanded.
Capital Allocation. During 2023, the Board played a significant role in steering the Company’s capital allocation strategy. Consistent with the Company’s announced strategy, this included investments in the pipeline, as discussed above, as well as new manufacturing facilities in the UK, Canada and Australia to support government partnerships, and investments to facilitate the commercialization of INT. The Board and the Science and Technology Committee played a key role in overseeing collaborations with other companies, including those that were announced with Immatics, CytomX and Life Edit Therapeutics. Lastly, the Board played a key role in overseeing our share buyback program and the decision in the first half of the year to curtail buybacks to preserve capital for future investments.
Executive Compensation and Talent Management. As discussed in greater detail elsewhere in this Proxy Statement, the Compensation and Talent Committee continues to update our compensation programs to ensure ongoing alignment between our executives and investors, including tying our performance-based programs to the Company’s strategy, evolving the weighting of different instruments in our equity programs, and evolving our culture and ways of working as we seek to scale our operations and prepare for multiple product launches while maintaining cost discipline. In addition, the Committee oversaw our talent management efforts, including performance, training and retention programs and the results of our second global pay equity analysis.
ESG Initiatives. The Board, acting primarily through the Nominating Committee, oversees Moderna’s efforts related to environmental, social and governance (ESG) matters. In 2023, this included launching a climate-based risk assessment and a description of any other agreement, arrangement, or understanding (including any short position or any borrowing or lending of shares),double-materiality assessment to prepare Moderna for potential disclosure requirements related to these matters. In addition, the effect or intent of which isCommittee oversaw efforts to mitigate lossimplement policies related to orESG, and increasing transparency on these matters, including reviewing our roadmap to manage the risk or benefit of share price changes for, or to increase or decrease the voting power of the nominee,
a description of all arrangements or understandings between or among the stockholder and each nominee and any other person or persons (naming such person or persons) pursuant to which the nomination is to be made by the stockholder or concerning the nominee’s potential service on the Board of Directors,
a written statement executed by the nominee acknowledging that as a director the nominee will owe fiduciary duties under Delaware law with respect to Moderna and its stockholders, and
allachieving publicly announced greenhouse gas reduction targets. For information, relating to such person that is required to be disclosed in solicitations of proxies for election of directors in an election contest pursuant to Regulation 14A under the Securities Exchange Act of 1934 (including such person’s written consent to being named in the proxy statement as a nominee and to serving as a director if elected).
The Nominating and Corporate Governance Committee may seek further information from or about the stockholder making the recommendation and the director candidate, including information about all business and other relationships between the director candidate and the stockholder. see “ESG Topics” below.
Our Corporate Governance Guidelines provide that at least a majority of the members of the Board must meet the independence standards prescribed by rules of The Nasdaq Stock Market. Our Board of Directors has determined that all current directors except Mr. Bancel, our Chief Executive Officer, are independent, as defined by the Securities and Exchange Commission (“SEC”)(SEC) and Nasdaq rules.
In making this determination, the Board considered the relationships that each non-employee director has with Moderna and other relevant facts and circumstances. In particular, our Board of Directors considered the association of our directors with entities that hold more than 5% of our common stock. There are no family relationships among any of our directors or executive officers.
Directors must notify the Chair of the Nominating Committee and Corporate Governance Committeeour Chief Legal Officer prior to accepting any new director roles or in connection with any significant change in employment status so we can fully assessthat the potential for conflicts or other factors that may compromise the director’s independence.ability to perform his or her duties may be fully assessed. Pre-clearance from the Chair of the Nominating Committee, acting on the advice of the Chief Legal Officer and in consultation with the full Nominating Committee, where appropriate, is required before a director may accept any such new roles. At least annually, the Board will evaluate all relationships between Moderna and each director in light of relevant facts and circumstances for the purpose of determining whether a material relationship exists that might signal a potential conflict of interest or otherwise interfere with such director’s ability to satisfy his or her responsibilities as an independent director.
 | 2024 Proxy statement | 17 |
Currently, theThe role of Chairman of the Board is separated from the role of Chief Executive Officer. Our Chief Executive Officer is responsible for recommending strategic decisions and capital allocation to the Board and for ensuring the execution of the recommended plans. The Chairman is responsible for leading the Board of Directors in its fundamental role of providing advice to and independent oversight of management. While ourOur bylaws and corporate governance guidelinesCorporate Governance Guidelines do not require that our Chairman and Chief Executive Officer positions be separate, our Board of Directors believes that having separate positions is appropriate for us at this time and demonstrates our commitment to good corporate governance.separate.
moderna 2021 Proxy statement |12
Board’s Role in Risk Oversight
Management is responsible for the day-to-day management of Moderna’s risks, while our Board of Directors, as a whole and through its committees, is responsible for overseeing risk management. In its risk oversight role, our Board of Directors must satisfy itself that the risk management processes designed and implemented by management are adequate and functioning as intended.
The Board exercises its oversight primarily through its committees. The full Board of Directors (or the appropriate committee in the case of risks that are under the purview of a particular committee) discusses with management our major risk exposures, their potential impact, and the steps we take to manage them. When a Board committee is responsible for evaluating and overseeing the management of a particular risk, the Chair of the relevant committee reports on the discussion to the full Board of Directors so the Board can coordinate the risk oversight role among the relevant parties.
Carrying out the duties and fulfilling the responsibilities of a director requires a significant commitment of time and attention. The Board recognizes that excessive time commitments can interfere with an individual’s ability to carry out and fulfill his or her duties effectively. In connection with its assessment of director candidates for nomination, the Board will assess whether the performance of any director has been or is likely to be adversely affected by excessive time commitments, including service on other boards.
Consistent with this belief, the Board amended its Corporate Governance Guidelines in 2020 to adopt overboarding limits.limits on the number of other boards on which directors may serve. Under the revised Guidelines, directors who also serve as executives of public companies should not serve on more than one board of a public company in addition to the Moderna board,Board, and other directors should not serve on more than three other boards of public companies in addition to the Moderna board,Board, absent special circumstances, such as a period of transition.
ApplicationtoDr.Langer.In applying these limits to the nomination ofDr. Langer. Dr. Langer is in compliance with our policy related to board service limits, and serves on the boards of two other public companies. The Nominating and Governance Committee considered the factnotes that while Dr. Langer previously served on four other public company boards prior(in addition to ours) at the adoptiontime our policy was adopted in 2020, in the last year he has come off two of the overboardingthose boards.
Application to Dr. Nabel. Dr. Nabel is also in compliance with our policy related to board service limits mentioned above. Dr. Langerand has also indicated he will not take on any new public board commitmentsbeen throughout her term. The Nominating Committee notes that would cause him to exceed the overboarding limits to the extent he comes off any other boards. In allowing for a transitiontemporary period, Dr. Nabel served as the Chief Medical Officer, part-time, for OPKO Health, while also serving on two public company boards (in addition to ours). In August 2023, Dr. Langer, the Committee took into account that he continues to make significant contributions to Moderna both insideNabel ceased acting as Chief Medical Officer for OPKO Health and outside Board and committee meetings, he has a perfect attendance record for Board and committee meetings in 2020, and he provides valuable insight as one of the Company’s largest shareholders and founding directors.
ApplicationtoMs.Tallett.In applying the overboarding limits to Ms. Tallett, the Nominating and Governance Committee noted that while Ms. Tallett currentlynow serves on four public boards in addition to the Moderna board, she will be retiring from one of those boards at its upcoming annual meeting, bringing her into compliance with the requirement by the end of May 2021. In addition, the Committee notedan Advisory Board for that Ms. Tallett also had perfect attendance for Board and committee meetings in 2020, and made substantial contributions to the Board in her first year, including taking on responsibility as Chair of the Audit Committee in January 2021.company.
Directors must notify the Chair of the Nominating Committee and Corporate Governance CommitteeChief Legal Officer in connection with accepting a seat on the board of directors of another business and obtain approval before joining so we can fully assess the potential for conflicts or other factors that may compromise the director’s ability to carry out his or her duties. See “Director Independence” above.
The Board does not believe that arbitrary limits on the number of consecutive terms a director may serve or on the directors’ ages are appropriate in light of the substantial benefits resulting from a sustained focus on Moderna’s business, strategy, and industry over a significant period of time.
moderna 2021 Proxy statement |13
We have adopted a Code of Ethics and Business Conduct and Ethics that applies to our Board of Directors and all of our officers and employees. In addition, we have adopted Corporate Governance Guidelines that formalize certain fundamental board policies and practices. Both of these documents are available on the “Investors—Corporate Governance”“Governance—Governance Documents” section of our Investor Relations website, https://investors.modernatx.com.investors.modernatx.com.
Since
 | 2024 Proxy statement | 18 |

 | 2024 Proxy statement | 19 |
We actively engage with our last Annual Meeting of shareholders throughout the year to solicit their views on our governance and compensation policies and practices, as well as current and emerging ESG trends. As our investor base has grown, we have updatedexpanded our investor outreach program and practices to seek the views of a broader range of investors, including those with a particular focus on socially responsible investing. Conversations throughout the year led by our Investor Relations team are supplemented by outreach with members of our Corporate Governance Guidelinesteam, senior leadership and other policies to implement the following changes:independent directors.
Following our 2023 Annual Meeting, we initiated contact for governance engagements with our 25 largest investors that are unaffiliated with our officers and directors, representing approximately 42% of our shares outstanding (as of September 2023), as well as several other investors who expressed an interest in meeting on governance topics. Including affiliated investors, this outreach included shareholders representing more than 55% of our shares outstanding. Governance outreach discussions focused on a number of topics that were voted on at the 2023 Annual Meeting, as well as other topics outlined below, including actions to adopt majority voting, implement proxy access, provide shareholders the right to call a special meeting, implement officer exculpation, and our current board structure and executive compensation programs.
|
| Executive Compensation Programs | ESG Initiatives | |
Director recruitment and Board composition • Board structure and independence • Majority voting • Proxy access • Special meeting right • Officer exculpation |
Our 2023 say-on-pay vote • Program structure, including the | |||
Transparency and disclosures on PSU goals |
| |||
|
Access to | |||
|
• Climate-related matters, including Scope 1, 2 and • Double-materiality analysis |
Board Composition and Governance. Investor feedback informed our decision to adopt majority voting and to adopt a proxy access bylaw—both of which were implemented in February 2024. Investor feedback also informed the proposal to amend our Certificate of Incorporation to grant shareholders the right to call a special meeting, and the decision to set the threshold at 20% of outstanding shares. This investor outreach also provided valuable feedback on the decision to pursue an amendment to our Certificate of Incorporation to allow for 2020
During 2020,officer exculpation, consistent with recent changes to Delaware law. Investor feedback has also informed our ongoing director search efforts as we assess the ongoing evolution of our Board of DirectorsDirectors.
Executive Compensation Programs. Investor feedback on our executive compensation programs was highly engagedconstructive, with support for our overall program design and emphasis on tying the experience of investors to results for our executives through programs that are weighted toward equity. Our Compensation and Talent Committee continues to incorporate this feedback into decisions around goal-setting and the structure of our compensation programs. We have also undertaken to provide greater visibility on the goals for our PSU awards in providing strategic oversightthis Proxy Statement.
ESG initiatives. Investor feedback on our ESG programs and transparency on these initiatives was quite positive. Most of our investors recognize Moderna’s important role in promoting access to medicines, particularly through efforts like our mRNA Access program and diverse enrollment targets in our clinical trials. Investors were also supportive of our efforts to developprovide greater transparency through our COVID-19 vaccine, overseeingESG Report (which includes SASB and GRI disclosures) and ESG Day. Investors appreciated our activitiesefforts to prepare to be a global commercial pharmaceutical company,disclose and obtain assurance on our Scope 1, 2 and 3 greenhouse gas emissions, as well as continuingour greenhouse gas reduction targets, and initiatives to oversee developments acrossassess climate risk and to conduct a double-materiality analysis.
Going forward, we will continue to engage with relevant stakeholders on these matters, continue to monitor the restESG reporting and disclosure landscape and assess additional ESG reporting frameworks. For more information, please see “ESG Topics.” In addition to our one-on-one discussions with investors, we host annual Investor Days throughout the year aimed at promoting understanding of our pipeline.
When the seriousness of the SARS-CoV-2 outbreak became clear in January 2020, our Board was engaged in the early decision to collaborate with the National Institutes of Health (“NIH”) in designing a vaccine, which quickly led to thescience and development of mRNA-1273, our COVID-19 vaccine. As the situation continued to evolve, it became increasingly clear that if Moderna’s vaccine candidate were to play a role in curbing the spread of SARS-CoV-2, additional capital would be needed to help fund our development efforts. The Board of Directors authorized an equity offering in February 2020, expecting that the use of some of the proceeds would enable the Company to have sufficient resources to further the development of a potential vaccine. On March 11, the World Health Organization (“WHO”) declared the spread of SARS-CoV-2 a pandemic,programs, including Vaccines Day and the dosing of the first patient in a Phase 1 clinical trial with mRNA-1273 occurred days later.
By early spring, much of the Company’s focus was trained on developing mRNA-1273, and the Board responded to the need for rapid evaluation of next steps and decision making by creating a COVID Committee of the Board of Directors. The COVID Committee met regularly throughout the rest of the year to review the Company’s development efforts for mRNA-1273. This Committee oversaw the preparations for and launch of further clinical trials and provided oversight of our efforts to scale our manufacturing capacity as we worked with contract manufacturing organizations to make an unprecedented product launch possible. The Committee brought in outside experts to supplement our directors’ existing expertise to advise on certain aspects of the vaccine’s development and possible launch.
Securing capital to fund the vaccine’s development was a significant focus, and at the Board’s direction, we undertook a second equity offering in May 2020. In addition, we agreed with BARDA in April and July that they would reimburse up to $955 million in costs related to the vaccine’s development, helping ensure that we could move quickly while also mitigating the financial risk associated with the vaccine’s development. Our Board and COVID Committee were also engaged in high-level decisions related to our clinical trials, including our decision in September 2020 to slow enrollment in the Phase 3 clinical trial to ensure that participants were representative of the diversity of the communities impacted by COVID-19. As we continued to receive favorable data from our clinical trials, our Board was also engaged in overseeing the at-risk scaling of our manufacturing, commercial and regulatory capabilities to facilitate the launch of mRNA-1273, both in the U.S. and abroad, should it receive regulatory authorization.
Within our standing committees of the Board, parallel efforts were undertaken to keep pace with these developments, as further described on the following pages. Our Nominating Committee had already taken aR&D Day.
moderna 2021 Proxy statement |14
significant step toward enhancing the Board’s oversight of a potential commercial launch through the additions of Dr. François Nader and Dr. Sandra Horning to the Board in late 2019 and early 2020, respectively. In July 2020, we welcomed Elizabeth Tallett to the Board, an experienced director with pharmaceutical industry and financial expertise. Ms. Tallett now chairs the Audit Committee, which has overseen an evolution in our financial reporting and our approach to risk management as we become a commercial company. Our Compensation Committee is overseeing a progression in our approach to talent management and compensation as we go through a rapid period of growth and commercialization. Lastly, our Product Development Committee continues to oversee our approach to vaccine developments for COVID-19 (including variants and boosters) while providing strategic oversight for the clinical development of our current and expanding pipeline.
Throughout the last year, the Board has remained engaged in ensuring that we also continue to look beyond COVID-19 and the current pandemic. As we look forward, the Board is focused on ensuring that we meet our first strategic priority of maximizing the impact of access to the Moderna COVID-19 Vaccine and value creation from this product. We believe this will lay the foundation for executing on our other strategic priorities, allowing to us continue to advance our Mission for patients and deliver value to our shareholders, our employees, our communities and our partners.
 | 2024 Proxy statement | 20 |
As described below, our Board of Directors has established fourfive committees: Audit, Compensation and Talent, Nominating and Corporate Governance, Product Development and Product Development.Science and Technology. The Board of Directors may establish other committees from time to time. All members of all fourthe five Board committees are independent directors.
The charterscharter for each of the Audit, Compensation and Talent, and Nominating and Corporate Governance Committees arecommittees is available on the “Investors—Corporate Governance”“Governance—Governance Documents” section of our Investor Relations website, https://investors.modernatx.com.

| Audit Committee | The Audit Committee’s responsibilities include: • appointing, approving the compensation of, and assessing the independence of our independent registered public accounting firm; • pre-approving • reviewing the overall audit plan with our independent registered public accounting firm and members of management responsible for preparing our financial statements; • reviewing and discussing with management and our independent registered public accounting firm our annual and quarterly financial statements and related disclosures, as well as the critical accounting policies and practices we use; • coordinating the oversight and reviewing the adequacy of our internal control over financial reporting; • establishing policies and procedures for the receipt and retention of accounting-related complaints and concerns; • • recommending, based upon review and discussions with management and our independent registered public accounting firm, whether our audited financial statements should be included in our Annual Report on Form 10-K; • monitoring the integrity of our financial statements and compliance with legal and regulatory requirements as they relate to our financial statements and accounting; • preparing the Audit Committee Report required by SEC rules to be included in our annual proxy statement; • reviewing all related person transactions for potential conflicts of interest and approving all appropriate transactions; • reviewing quarterly earnings • discussing the guidelines and policies that govern the process by which the Company’s exposure to risk is assessed and managed by management; and • exercising general oversight over the Company’s information security and technology risks, including the Company’s information security and related risk management programs. |
Members: • Ms. Tallett • Mr.
• Mr. Sagan | ||
Meetingsin Independence: Financial experts: | ||
Representative, recent discussion topics
| • | Financial reporting and significant accounting items, including as a result of our shift to a commercial market and in connection with the resizing of our manufacturing footprint | |
| •
| Ongoing enhancements to our cybersecurity program, including onboarding of |
| • | Ongoing evolution of our internal audit, tax and | |
| • | Capital allocation strategy, including approach to investments, tax and oversight of |
 | 2024 Proxy statement | 21 |
Overview of plans for developing the company’s compliance and commercial organizations as part of the launch of mRNA-1273
Enterprise risk management
Cybersecurity
Updates to investment strategies and preservation of capital
moderna 2021 Proxy statement |15
 | Compensation and Talent Committee | The Compensation and Talent Committee’s responsibilities include: • reviewing and establishing our overall management compensation philosophy and policy; • annually reviewing and recommending to the Board evaluating the CEO’s performance, • approving the cash and equity compensation of our other executive officers; • overseeing the operation of all compensation plans, policies and • reviewing the Company’s •
• appointing and overseeing compensation consultants and other advisors retained by the • reviewing and recommending to the Board of Directors the compensation of our directors; and • preparing the Compensation Committee Report required by SEC rules to be included in our annual proxy statement. |
Members: • Mr. •
• Ms.Tallett | ||
Meetingsin Independence: | ||
Representative, recent discussion topics
| • | Ongoing evolution of our executive compensation program, including the weighting of equity plans, goal-setting and the ties between our pay programs and strategy to ensure alignment with investors | |
| •
| Development of our future operating model as we seek to grow sustainably and |
| • | Maintaining Moderna’s culture and Mindsets | |
| • | Talent development efforts, including retention, development and performance management | |
| • | Global pay practices and the results of our second annual pay equity analysis | |
| • | Succession planning for
|

| Nominating and Corporate | The Nominating and Corporate Governance Committee’s responsibilities include: •
•
reviewing the composition of the Board •
reviewing and recommending corporate governance practices to the • overseeing the evaluation of our • reviewing |
| ||
initiatives, such as political engagement, actions to mitigate risks related to climate change, as well as philanthropic initiatives, such as the Moderna Charitable Foundation. | ||
Governance Committee Members: • • •
| ||
| Meetings in 2023: 4 | ||
Representative, recent discussion topics
| • | Evolution of the Company’s governance practices to implement majority voting, proxy access, a special meeting right, and officer exculpation, and reviewing investor feedback on these topics | |
| • | Board succession planning and director recruitment | |
| • | Rotation of committee leadership roles | |
| •
| Updates to | |
| • | Expansion and evolution of ESG efforts, including
|
 | 2024 Proxy statement | 22 |
moderna 2021 Proxy statement |16
 | Product Development | The Product Development Committee’s responsibilities include: • assessing our product development strategy; • reviewing product development plans for our • evaluating management recommendations • reviewing R&D- and pipeline-related goals in performance-based compensation plans; • providing guidance and assisting in assessments of scientific talent; and • advising the Board on scientific and R&D aspects of licensing, strategic partnerships and acquisition or divestiture transactions. |
Committee Members: • Dr. Horning (Chair) • • Dr. • Dr. Nader
| ||
| Meetings in 2023: 7 | ||
Representative, recent discussion topics
| • | Advancement of our late-stage pipeline, including design and launch of pivotal trials for seasonal flu, next-generation COVID, and combination vaccine for COVID + seasonal flu | |
| • | Ongoing clinical results from our Phase 2b trial for INT (melanoma), and the launch of Phase 3 trials of INT for melanoma and non-small cell lung cancer | |
| • | R&D- and pipeline-related goals included in 2024 performance-based compensation plans |
 | Science and Technology |
• reviewing, evaluating and advising the Board regarding platform development, including advances in • identifying significant emerging science and technology issues and trends relevant to our mRNA platform; • reviewing, evaluating and advising the Board regarding potential new modalities and strategies to expand the utility of existing modalities; and • reviewing and advising the Board regarding our intellectual property strategies. |
Committee Members: • • •
| ||
| Meetings in 2023: 5 | ||
Representative, recent discussion topics
| • | Oversight of key external collaborations and investments with outside partners | |
| • | Platform research strategy and budget | |
| • | Strategy related to intellectual property and licensing |
Each director is expected to make reasonable efforts to attend all Board and applicable committee meetings. Attendance rates will beis taken into account by the Nominating and Corporate Governance Committee and the Board when they assess directors for re-nomination to the Board. Each director is also expected to attend our annual shareholder meetings, and all of our directors attended the 2023 Annual Meeting of Shareholders.
The full Board of Directors met 13five times in formal meetings during 2020,2023, in addition to holding frequent calls and informal sessions. Each director attended (virtually or in person) at least 96%75% of the aggregate meetings of the Board (held while such person was a director) and meetings held by all committees of the Board on which such person served.
The non-management directors meet at regularly scheduled executive sessions without management participation, and executive sessions with only independent directors are held regularly. We recently amended our Corporate Governance Guidelines to require these sessions to be held at least three times per year. When theparticipation. The Chair of the Board is a non-management director and an independent director (as is currently the case), then the Chair of the Boardand presides at theseall Board meetings. If the Chair of the Board is not independent, then the director who presides at these meetings will be chosen by the non-management directors.
Director Onboarding and Continuing Education
Moderna conducts an orientation program for each new director to familiarize that individual with our business and strategic plans, key policies and practices, principal officers and management structure, auditing and compliance processes, and Code of Business Conduct and Ethics. The Nominating and Corporate Governance Committee is responsible for periodically providing materials or briefing sessions for continuing directors on topics that will assist them in discharging their duties.
 | 2024 Proxy statement | 23 |
In practice, our Board has conducted a self-evaluation annually, and in 2020 we updatedPursuant to our Corporate Governance Guidelines, our Board has committed to require an annualconduct a self-evaluation (rather than committing to do so only periodically). The Board self-evaluation is designedat least annually to assess whether the Board and its committees are functioning effectively. IndividualOur Board committees may conduct self-evaluations for the same purpose.periodically to assess whether they are functioning effectively. Any such evaluation will consider the performance of the Board or the committee, as the case may be, as a unit (rather than the performance of any individual director). The Nominating and Corporate Governance Committee oversees ourthe Board’s annual self-evaluation process.
In 2020,2023, the Board’s self-evaluation wasutilized an independent third party who conducted late in the summer by an outside third-party who conducteda survey and individual interviews with each of our directorsdirector regarding the Board’s operations. The reviewer then providedoperations before reporting back to full Board of Directors. As a report outresult of the feedback from this evaluation, updates were made to the entire Board at a meeting in fall 2020composition of the Board’s committees and leadership roles were rotated. Additional changes were implemented to reviewpromote the Board’s effectiveness and discuss the feedback.
to increase internal communications, among other changes. In the past, these self-evaluations have similarly informed decisions regarding Board operations and Board composition, which have been acted upon by the Nominating and Corporate Governance Committee.
moderna 2021 Proxy statement |17
Compensation Committee Interlocks and Insider Participation
None of the members of our Compensation and Talent Committee has at any time during the prior three years been one of our officers or employees. None of our executive officers currently serves, or in the past fiscal year has served, as a member of the board of directors or compensation committee of any entity that has one or more executive officers serving on our boardBoard of directorsDirectors or compensation committee.Compensation and Talent Committee.
Moderna conducts an orientation program for each new director to familiarize that individual with our business and strategic plans, key policies and practices, principal officers and management structure, auditing and compliance processes, and Code of Business Conduct and Ethics.
Any person whether or not an employee,who wishes to contact the Board can do so:
| • | By e-mail to ir@modernatx.com; or |
| • | By reaching our Corporate Secretary as described on page 87 under “Information about the 2024 Annual Meeting of Shareholders—How can I contact the Corporate Secretary?”. |
Any person who has a concern about Moderna’s conduct, including with respect to accounting, internal accounting controls, or auditing matters, may, in a confidential or anonymous manner, communicate that concern to our Compliance Officer:
| • | By e-mail to ComplianceOfficer@modernatx.com (anonymity cannot be maintained); |
| • | In writing (which may be done anonymously), by mail to Moderna, Inc., Attention: Compliance Officer, 200 Technology Square, Cambridge, Massachusetts 02139; |
| • | Online at https://moderna.whispli.com/compliancehotline(which may be done anonymously); or |
| • | By calling the Compliance Hotline at 844-971-2551. |
Any person who is the designated contact for these purposes. Contact may be made:
By e-mail to ComplianceOfficer@modernatx.com (anonymity cannot be maintained);
In writing (which may be done anonymously), by mail to Moderna, Inc., Attention: Compliance Officer, 200 Technology Square, Cambridge, Massachusetts 02139;
Online at https://www.whistleblowerservices.com/Moderna (which may be done anonymously); or
By calling the Compliance Hotline at 866-265-3948, which is managed by a third-party that is required to maintain the anonymity of the caller if so requested.
If you wishwishes to communicate directly with the Audit Committee you may do so:
In writing (which can be done anonymously), by mailso through the channels mentioned above, directing their communication to Moderna, Inc., Attention:the attention of the Chair of the Audit Committee.
 | 2024 Proxy statement | 24 |
The Nominating Committee 200 Technology Square, Cambridge, Massachusetts 02139;
Online at https://www.whistleblowerservices.com/Moderna (which maywelcomes recommendations from shareholders for director candidates. Shareholder-recommended candidates will be done anonymously); or
By callingevaluated in the Compliance Hotline and askingsame manner as candidates that the matter be forwardedcome to the chairperson of the Audit Committee. committee’s attention from other sources.
moderna 2021 ProxyTo recommend a director candidate, a shareholder should send the following information to our Corporate Secretary as described on page 87 under “Information about the 2024 Annual Meeting of Shareholders—How can I contact the Corporate Secretary?”:
| • | such shareholder’s name and address of record; |
| • | a representation that such shareholder is a record holder of the Company’s securities, or if the shareholder is not a record holder, evidence of ownership in accordance with Rule 14a-8(b)(2) of the Securities Exchange Act of 1934, as amended (the Exchange Act); |
| • | the candidate’s name, age, business and residence address; |
| • | the educational background, current principal occupation or employment, and principal occupation or employment for the preceding five full years of the candidate; |
| • | a description of the qualifications and background of the candidate, which address the minimum qualifications and other criteria for Board membership approved by the Board; |
| • | a description of all arrangements or understandings between the shareholder and the candidate; and |
| • | all information relating to such candidate that is required to be disclosed in solicitations of proxies for election of directors in an election contest pursuant to Regulation 14A under the Exchange Act (including such person’s written consent to being named in the proxy statement as a nominee and to serving as a director if elected). |
The Nominating Committee may seek further information from or about the shareholder making the recommendation and the recommended director candidate, including information about all business and other relationships between the director candidate and the shareholder. Any shareholder recommendation for a director candidate must be submitted to the Company not less than 120 calendar days prior to the date on which the Company’s proxy statement |18
Backwas released to Contentsshareholders in connection with the previous year’s annual meeting.
 | 2024 Proxy statement | 25 |
Our non-employee directors are eligible to receive the following cash retainers, which are proratedpro-rated for partial years of service:partial-year service or time as Committee chairs, and are paid quarterly:
|
|
|
Annual Retainer for service on the Board of Directors | $ | 50,000 |
Additional Annual Retainer for service as: |
|
|
Non-Executive Chairman of the Board of Directors | $ | 40,000 |
Chair of the Audit Committee | $ | 25,000 |
Member of the Audit Committee (other than Chair) | $ | 10,000 |
Chair of the Compensation & Talent Committee | $ | 20,000 |
Member of the Compensation & Talent Committee (other than Chair) | $ | 10,000 |
Chair of the Nominating and Corporate Governance Committee | $ | 10,000 |
Member of the Nominating and Corporate Governance Committee (other than Chair) | $ | 5,000 |
Chair of the Product Development Committee | $ | 15,000 |
Member of the Product Development Committee (other than Chair) | $ | 7,500 |
| Annual Retainer for service on the Board of Directors | $ | 80,000 |
| Additional Annual Retainer for service as: | ||
| Non-Executive Chairman of the Board of Directors | $ | 50,000 |
| Committee Chair | $ | 20,000 |
Upon initial election to our Board of Directors, each non-employee director is granted stock optionsan equity award with an aggregate targeted grant date fair value of $400,000 (the “Initial Grant”)Initial Grant). TheseBeginning in April 2021, 75% of the value of each Initial Grant is delivered in stock options and 25% is delivered in restricted stock units (RSUs). The stock option and RSU portions of the Initial Grant vest in full on the one-year anniversary of the grant date if the recipient has continuously served as our director for that year. Dr. Nabel was a member of our Board of Directors from January to July 2020 and was reappointed to the Board in March 2021. In light of the fact that Dr. Nabel previously served on the Board, she and the Board agreed that she would not receive an Initial Grant upon being reappointed as a director.
On the date of each succeeding annual meeting of stockholders,shareholders, each continuing non-employee director is granted stock optionsan equity award with an aggregate targeted grant date fair value of $425,000 (the “Annual Grant”)Annual Grant). TheseSince April 2022, our directors have had the ability to choose to have the value of each Annual Grant delivered solely as stock options or in the form of a mix of RSUs and stock options as chosen by each non-employee director, provided that no more than 25% of such value may be delivered in the form of RSUs. The option and RSU portions of the Annual Grant vest in full on the earlier of the one-year anniversary of the grant date and the next annual meeting of stockholders,shareholders, if the recipient has continuously served as a director through the applicable vesting date.
The options subject to the Initial Grant and the Annual Grants havestock option portion of each director equity grant has an exercise pricesprice per share equal to the closing price of a share of Moderna’s common stock on the date of grant, and termsan expiration period of ten years.years from the grant date. The number of stock options granted for any director equity grant is determined by dividing the value attributable to the stock option award by the product of (i) the average closing stock price over the preceding 20-trading days up to and including the last trading day immediately preceding the grant date and (ii) the Black-Scholes ratio, which is calculated using the Black-Scholes value of a stock option on the grant date divided by the closing stock price on the effective date of grant. The number of RSUs granted for any director equity grant is determined by dividing the value attributable to the RSU award by the average closing stock price over the preceding 20-trading days up to and including the last trading day immediately preceding the grant date. If a new non-employee director joins our Board of Directors between annual meetings of stockholders,shareholders, then such non-employee director will be granted a pro-rata portion of the Annual Grant based on the time between such director’s appointment and our next annual meeting of stockholders.shareholders. The Initial Grants and Annual Grantsdirector equity grants are subject to full accelerated vesting upon a “sale event,” as defined in the Company’s 2018 Stock Option and Incentive Plan, as amended from time to time (the “2018 Plan”)2018 Stock Plan).
The aggregate amount of cash and equity compensation paid to any non-employee director in a calendar year may not exceed $1,500,000 for the first year of service and $1,000,000 for each year of service thereafter (or such other limits as may be set forth in the 2018 Stock Plan or any similar provision of a successor plan).
Employee directors receive no additional compensation for their service as a director.
We will reimburse all reasonable out-of-pocket expenses incurred by directors for their attendance at meetings of our Board of Directors or any committee thereof. We have in the past provided, and may in the future provide, security services to our directors.
moderna 2021 Proxy statement |19
 | 2024 Proxy statement | 26 |
Our Stock Ownership Policy provides that on or before December 31, 2024, or the fifth anniversary of an individual director’s appointment to the Board (whichever is later), each non-employee director must own shares of Moderna’s common stock equal to at least six times the amount of the annual cash retainer paid for regular service on the Board, exclusive of committee fees (i.e., $300,000$480,000 of Moderna stock). For more information, see “—“Equity-Related Policies and Practices—Non-Employee Director and Executive Officer Stock Ownership Policy,” below.
The following table provides information regarding the total compensation that was earned by or paid to each of our non-employee directors during the year ended December 31, 2020.2023. Mr. Bancel, our Chief Executive Officer, did not receive any additional compensation for his service as a director. Mr. Bancel’s compensation is discussed in the Compensation Discussion &and Analysis and compensation tables, which beginsbegin on page 29.38. The amounts below for Option Awards and Stock Awards reflect the grant date fair value of these awards, which, together, are lower than the target value due to the fact that the number of RSUs and stock options granted was calculated based upon the 20-trading day trailing average closing price prior to grant, as described under “—Equity Grants,” above.
Name | Fees Earned or Paid in Cash ($) | Option Awards ($)(1) | All Other Compensation ($) |
| Total ($) | ||||
Noubar B. Afeyan, Ph.D.(2) | $ | 107,500 | $ | 425,000 | $ | — |
| $ | 532,500 |
Stephen Berenson(3) |
| 78,750 |
| 425,000 |
| — |
|
| 503,750 |
Sandra Horning, M.D.(4) |
| 46,875 |
| 825,000 |
| — |
|
| 871,875 |
Robert Langer, Sc.D.(5) |
| 55,000 |
| 425,000 |
| 20,000 | (6) |
| 500,000 |
Elizabeth Nabel, M.D.(7) |
| 49,375 |
| 425,000 | (7) | — |
|
| 474,375 |
François Nader, M.D.(8) |
| 60,000 |
| 425,000 |
| — |
|
| 485,000 |
Israel Ruiz(9) |
| 40,625 |
| — |
| — |
|
| 40,625 |
Paul Sagan(10) |
| 80,625 |
| 425,000 |
| — |
|
| 505,625 |
Moncef Slaoui, Ph.D.(11) |
| 32,500 |
| 425,000 | (11) | — |
|
| 457,500 |
Elizabeth Tallett(12) |
| 35,000 |
| 754,167 |
| — |
|
| 789,167 |
(1) The amounts reported represent the aggregate grant date fair value of the stock options awarded to the non-employee directors in the year ended December 31, 2020, calculated in accordance with FASB ASC Topic 718. Such grant date fair values do not take into account any estimated forfeitures. The assumptions used in calculating the grant date fair value of the stock options reported in this column are set forth in Note 14 to our Consolidated Financial Statements for the year ended December 31, 2020 included in our Annual Report. The amounts reported in this column reflect the accounting cost for these stock options and do not correspond to the actual economic value that may be received by the non-employee directors upon the exercise of the stock options or any sale of the underlying shares of common stock. (2) As of December 31, 2020, Dr. Afeyan held outstanding options to purchase a total of 155,538 shares of our common stock, 137,168 of which were vested. Dr. Afeyan is affiliated with Flagship Pioneering, Inc. and prior to 2018, Flagship Pioneering, Inc. was granted equity for Dr. Afeyan’s service on our Board of Directors. As of December 31, 2020, Flagship Pioneering, Inc. held options to purchase a total of 33,116 shares of our common stock that were issued for such service. See “Security Ownership of Certain Beneficial Owners and Management” for additional information regarding Flagship Pioneering’s and its affiliated entities’ beneficial ownership of our common stock. (3) As of December 31, 2020, Mr. Berenson held options to purchase a total of 155,438 shares of our common stock, 137,168 of which were vested. (4) Dr. Horning was elected to our Board on April 29, 2020 and received an initial equity grant upon joining the Board in addition to the annual equity award granted to all continuing directors at the time of the Annual Meeting. As of December 31, 2020, Dr. Horning held options to purchase 35,466 shares of our common stock, none of which were vested. (5) As of December 31, 2020, Dr. Langer held options to purchase a total of 284,006 shares of our common stock, 265,736 of which were vested. (6) The amount reported represents $20,000 in consulting fees for Dr. Langer’s service as a member of our Scientific Advisory Board (the “SAB”) pursuant to a Scientific Advisory Board Member Agreement between the Company and Dr. Langer, dated as of September 19, 2014. Under such agreement, Dr. Langer is provided with a quarterly consulting fee of $5,000 in exchange for his attendance at SAB meetings and guidance in the field of research, development and commercialization of products. (7) Dr. Nabel resigned from the Board on July 30, 2020. As of December 31, 2020, Dr. Nabel did not hold any options to purchase shares of our common stock. Dr. Nabel forfeited her annual equity award for 2020 upon her resignation. (8) As of December 31, 2020, Dr. Nader held options to purchase a total of 75,879 shares of our common stock, 57,609 of which were vested. (9) Mr. Ruiz did not stand for re-election to the Board and served as a director through April 29, 2020. As of December 31, 2020, Mr. Ruiz did not hold any options to purchase shares of our common stock. (10) As of December 31, 2020, Mr. Sagan held options to purchase a total of 109,689 shares of our common stock, 91,419 of which were vested. (11) Dr. Slaoui resigned from the Board on May 15, 2020, upon being appointed as the Chief Scientific Advisor to the White House’s Operation Warp Speed initiative. As of December 31, 2020, Dr. Slaoui did not hold any options to purchase shares of our common stock. Dr. Slaoui forfeited his annual equity award for 2020 upon his resignation. (12) Ms. Tallett was elected to our Board on July 1, 2020 and received an initial equity grant upon joining the Board in addition to a pro rata annual equity award for 2020 and the period in 2021 prior to the annual meeting of shareholders. As of December 31, 2020, Ms. Tallett held options to purchase 23,819 shares of our common stock, none of which were vested. | |||||||||
| Name | Fees Earned or Paid in Cash | Stock Awards(1)(2) | Option Awards(1) | All Other Compensation | Total | ||||||||||
| Noubar Afeyan, Ph.D.(3) | $ | 150,000 | $ | — | $ | 381,269 | $ | 132,250(4) | $ | 663,519 | |||||
| Stephen Berenson(5) | 90,000 | 95,262 | 285,983 | — | 471,245 | ||||||||||
| Sandra Horning, M.D.(6) | 100,000 | — | 381,269 | — | 481,269 | ||||||||||
| Robert Langer, Sc.D.(7) | 80,000 | — | 381,269 | — | 461,269 | ||||||||||
| Elizabeth Nabel, M.D.(8) | 95,000 | 95,262 | 285,983 | — | 476,245 | ||||||||||
| François Nader, M.D.(9) | 100,000 | — | 381,269 | — | 481,269 | ||||||||||
| Paul Sagan(10) | 80,000 | — | 381,269 | — | 461,269 | ||||||||||
| Elizabeth Tallett(11) | 100,000 | — | 381,269 | — | 481,269 | ||||||||||
| (1) | The amounts reported represent the aggregate grant date fair value of the RSUs and stock options awarded to the non-employee directors in the year ended December 31, 2023, calculated in accordance with Financial Accounting Standards Board (FASB) Accounting Standards Codification (ASC) Topic 718. Such grant date fair values do not take into account any estimated forfeitures. The assumptions used in calculating the grant date fair value of the equity awards reported in these columns are set forth in Note 12 to our Consolidated Financial Statements for the year ended December 31, 2023, included in our Annual Report. The amounts reported in these columns reflect the grant date fair value for each award, and, together, differ from the targeted amounts for the Annual Grant of $425,000, due to the fact that a 20-trading day trailing average closing price convention is used for calculating the number of RSUs and stock options granted. |
| (2) | As of December 31, 2023, each non-employee director who selected RSUs as part of their annual equity award held 732 unvested RSUs. |
| (3) | As of December 31, 2023, Dr. Afeyan held outstanding options to purchase a total of 171,068 shares of our common stock, 164,934 of which were vested. Dr. Afeyan is affiliated with Flagship Pioneering, Inc. and prior to 2018, Flagship Pioneering, Inc. was granted equity for Dr. Afeyan’s service on our Board of Directors. As of December 31, 2023, Flagship Pioneering, Inc. held options to purchase a total of 33,116 shares of our common stock that were issued for such service. See “Security Ownership of Certain Beneficial Owners and Management” for additional information regarding Flagship Pioneering’s and its affiliated entities’ beneficial ownership of our common stock. |
| (4) | The amount reported represents incremental costs borne by the Company for the provision of security services to Dr. Afeyan in response to the heightened threat environment faced by individuals associated with our Company. |
| (5) | As of December 31, 2023, Mr. Berenson held options to purchase a total of 168,181 shares of our common stock, 163,580 of which were vested. |
| (6) | As of December 31, 2023, Dr. Horning held options to purchase a total of 49,742 shares of our common stock, 43,607 of which were vested. |
| (7) | As of December 31, 2023, Dr. Langer held options to purchase a total of 299,636 shares of our common stock, 293,502 of which were vested. |
| (8) | As of December 31, 2023, Dr. Nabel held options to purchase a total of 13,882 shares of our common stock, 9,281 of which were vested. |
| (9) | As of December 31, 2023, Dr. Nader held options to purchase a total of 68,409 shares of our common stock, 62,275 of which were vested. |
| (10) | As of December 31, 2023, Mr. Sagan held options to purchase a total of 125,319 shares of our common stock, 119,185 of which were vested. |
| (11) | As of December 31, 2023, Ms. Tallett held options to purchase 39,449 shares of our common stock, 33,315 of which were vested. |
moderna 2021 Proxy statement |20
 | 2024 Proxy statement | 27 |
Certain Relationships and Related Party Transactions
Other than the ordinary course compensation agreements described under the sections entitled “Director Compensation” and “Compensation Discussion &and Analysis” and the transactions described below, for the year ended December 31, 2020,since January 1, 2023, there has not been and there is not currently proposed, any transaction or series of similar transactions to which we were, or will be, a party in which the amount involved exceeded, or will exceed, $120,000 and in which any director, executive officer, holder of five percent or more of any class of Moderna’s capital stock, or any member of the immediate family of, or entities affiliated with, any of the foregoing persons, had, or will have, a direct or indirect material interest.
Prior to our IPO, we entered into a second amended and restated Investor Rights Agreement with certain holders of our stockholders.shareholders. The Investor Rights Agreement providesprovided the holders of approximately 61.144 million shares of our common stock rights with respect to the registration of those shares under the Securities Act of 1933, as amended (the Securities Act), including demand registration rights, short-form registration rights, and piggyback registration rights.
Certain holders Each of our common stock are entitled to demand registration rights. We will be required, upon the written request of a majority of holders of these shares of our common stock to file a registration statement and to use commercially reasonable efforts to effect the registration of all or a portion of these shares for public resale. We are required to effect only two registrations upon the request of a majority of holders.
Short-form registration rights
Certain holders of our common stock are entitled to short-form registration rights. If we are eligible to file a registration statement on Form S-3, then upon the written request of 20% in interest of these holders to sell registrable securities at an aggregate price of at least $2.5 million, we will be required to use commercially reasonable efforts to effect a registration of such shares. We are required to effect only two registrations in any twelve-month period.
If we register any of our securities, either for our own account or for the account of other security holders, the holders of these shares are entitled to include their shares in the registration. Subject to certain exceptions, Moderna and the underwriters may limit the number of shares included in the underwritten offering to the number of shares that we and the underwriters determine in our sole discretion will not jeopardize the success of the offering.
Expiration of registration rights
The demand registration rights and short-form registration rights granted under the Investor Rights Agreement will terminate on the earlier to occur ofterminated in December 11, 2023, or, as to each holder, at such earlier time that such holder (i) can sell all shares held by it in compliance with SEC Rule 144(b)(1)(i) or (ii) holds 1% or less of our common stock, and all registrable securities held by such holder can be sold in any three-month period without registration in compliance with SEC Rule 144.2023.
We have entered into agreements to indemnify our directors and executive officers. These agreements will, among other things, require us to indemnify these individuals, to the maximum extent allowed under Delaware law, for certain expenses (including attorneys’ fees), judgments, fines, and settlement amounts they reasonably incur in any action or proceeding, including any action by or in our right, on account of any services undertaken by such person on behalf of Moderna or that person’s status as a member of our Board of Directors.
moderna 2021 Proxy statement |21
Policies for Approval of Related Party Transactions
We have adopted a written policy providing that our Audit Committee is responsible for reviewing and overseeing related party transactions. For purposes of this policy, a related person is defined as (i) any Moderna director or executive officer, (ii) any director nominee, (iii) security holders known to us to beneficially own more than five percent of any class of Moderna’s voting securities, or (iv) the immediate family members of any of such persons. In reviewing any related party transaction, the Audit Committee will take into account, among other factors that it deems appropriate, whether the transaction is on terms no less favorable to Moderna than terms generally available in a transaction with an unaffiliated third-party under the same or similar circumstances and the extent of the related person’s interest in the transaction.
The Audit Committee reviewed the following transactions that took place between Moderna and related persons since January 1, 2020:
 | 2024 Proxy statement | 28 |
Ms. Tallett’s son was employed by Moderna until October 2020as a senior director in our finance organization. He was employed in the role prior to Ms. Tallett’s appointment to the Board in July 2020, and he resigned voluntarily from the position. For 2020, his total compensation (including salary, bonus and the grant date fair value of equity awards) was approximately $361,000, and was established in accordance with the Company’s employment and compensation practices applicable to employees with equivalent qualifications and responsibilities and holding similar positions.
Dr. Nabel was the President of Brigham Healththrough February 2021, and one of its affiliated hospitals— Brigham and Women’s Hospital—was one of 99 clinical trial sites selected by the NIH for the Phase 3 study of mRNA-1273. To alleviate any potential concern about the conduct or outcome of the vaccine trials, Dr. Nabel resigned from our Board in July 2020, prior to the commencement of the clinical trial, and did not rejoin the Board until after she stepped down from Brigham Health.
The Audit Committee determined that these transactions did not impact the independence of Ms. Tallett or Dr. Nabel, respectively.
moderna 2021 Proxy statement |22
At Moderna, our commitment toEnvironmental, Social and Governance (ESG) strategy and corporate citizenship issocial responsibility program are built on a foundation of integrity, quality and respect. These values provide solid footinga foundation for the creationus to build and support of long-term programs that focus ondemonstrate our commitment to patients, employees, the environment and our local communitiescommunities. Our Nominating and ethics.Corporate Governance Committee oversees ESG matters and practices. The committee reports to the full Board on ESG matters and our progress on sustainability initiatives. Our Chief Legal Officer, reporting to our CEO, leads our ESG strategy, with Executive Committee members overseeing additional elements of particular ESG initiatives. Included below is a description of a fewseveral topics that we view as key to promoting our long-term sustainability.value and impact. In June 2023, we published our second ESG Report, which provides further detail on these initiatives. Our ESG Report can be found on our website at http://www.modernatx.com under the “Responsibility—Corporate policies” section. Additionally, we hosted our second ESG Day in December 2023, where we discussed with investors our corporate mission and key focus areas for our corporate responsibility strategy. During 2023, we also initiated a double-materiality analysis to inform anticipated disclosure requirements and to validate our understanding of where we can create positive long-term value for our stakeholders.
Moderna’s mission is to Medicines
deliver the greatest possible impact to people through mRNA medicines. Our priority as a company that has just launched its firstrelatively new commercial productcompany is to continue to accelerate the development of safe and effective mRNA medicines for patients.medicines. To do so, we undertake sustained, long-term investment in technology creation.
Additionally, We expect our ongoing investment in our preclinical and clinical research engine willand development to help us continue to advance potentially life-changing therapeutics and vaccines where there are few or, in many cases, no treatment options for patients. These areas include infectious diseaserespiratory vaccines, public healthlatent and emerging vaccines, and rare disease indications.therapeutics, and oncology therapeutics.
In 2020,2022, we first articulated our global health strategy, which is centered on three key pillars: developing vaccines against priority pathogens, mRNA Access™ and regional manufacturing. We continue to focus on developing vaccines against priority pathogens identified in the World Health Organization’s (WHO) R&D Blueprint, and those identified by the Coalition for Epidemic Preparedness Innovations (CEPI). Our objective remains to create a portfolio of vaccines that are able to leverage our scale of manufacturing and the growing safety database to more rapidly respond to outbreaks in the future. We have advanced clinical candidate vaccines against COVID-19, Zika, chikungunya, HIV, Mpox, Nipah and pandemic influenza, many through public-private partnerships.
Our mRNA Access program enables researchers around the world to utilize our mRNA technology platform played an important roleto pursue research in supporting a rapid responsetheir own labs on emerging and neglected infectious diseases. During 2023, the number of institutions collaborating with us through mRNA Access nearly doubled, to 16. In addition, we continue to advance regional manufacturing plants in Australia, Canada and the UK, in part to prepare for future pandemics. Further detail on our global pandemic, andhealth strategy is included in our ESG Report.
Prior to authorization of our first commercial product, our COVID-19 vaccine, we announced in December 2020 our commitment to access to vaccines and therapeutics. This commitment is now authorizedreflected in the following principles:
| • | We are committed to developing a broad portfolio of vaccines and therapeutic solutions to address epidemiological challenges worldwide. |
| • | We will invest in R&D in areas of unmet need. |
| • | We will work to include communities that have been under-represented in clinical research in our development programs, as well as those who are disproportionately impacted by the respective disease. |
| • | We aim to provide effective and affordable vaccines and therapeutics to all populations. |
| • | We will price our products through differential pricing frameworks. |
| • | We are committed to participating in key public-private partnerships. |
| • | GAVI-eligible countries will get our lowest prices, and we commit to an annual independent third-party audit of this commitment. |
 | 2024 Proxy statement | 29 |
In 2022, as part of our continued support for achieving global health equity, we updated our COVID-19 patent pledge to provide that we will never enforce our patents for COVID-19 vaccines against manufacturers in low- and middle-income countries in the Gavi COVAX Advance Market Commitment, provided that the manufacturers produce vaccines solely for use in more than 30those countries. We are actively participatingIn non-AMC 92 countries, we remain willing to license our technology for COVID-19 vaccines to manufacturers in discussions with multilateral organizations, such as COVAX,those countries on commercially reasonable terms, which will enable us to help expand access and protect populations around the world.
Just as we workedcontinue to ensure representation of communities of color and vulnerable populationsinvest in the Phase 3 clinical trial of our COVID-19 vaccine (with 37% of participants coming from diverse communities), we have an ongoing commitmentresearch to increasing diversity in our clinical trials.
We believe it is our responsibility as an industry, as a company and as global citizens to help ensure a pandemic of this magnitude never happens again. Moderna has already launched a clinical program to boost immunity to emerging variants of SARS-CoV-2, as we advance other infectious diseasedevelop new vaccines, and therapiesprepare for the preventionnext pandemic and treatmentmeet other pressing areas of infectious disease threats that can place millions of people at risk around the world.unmet medical need.
We are also partnering with foundations, government organizations and universities to develop mRNA solutions to critical global public health challenges. This includes a new partnership with the International AIDS Vaccine Initiative and the Bill & Melinda Gates Foundation to accelerate human validation of novel HIV vaccination strategies, as well as a second mRNA-based approach to HIV vaccination in collaboration with the National Institutes of Health. We also have programs to develop a Zika vaccine and a vaccine against the Nipah virus, a zoonotic virus transmitted to humans from animals that is on the WHO’s Blueprint list of epidemic threats needing urgent R&D action
Our pipeline also includes potential therapeutics to address rare diseases, such as propionic acidemia (PA), methylmalonic acidemia (MMA), phenylketonuria, glycogen storage disease type 1a (GSD1a), and autoimmune diseases that cannot behave not been addressed bythrough traditional approaches. PartnershipsDuring 2023, our most advanced programs for rare diseases, PA and MMA, demonstrated the potential of our platform to address rare diseases. We expect to advance these two programs to pivotal trials in 2024. We have ongoing collaborations with Vertex Pharmaceuticals and theto apply mRNA technology to addressing cystic fibrosis, with Chiesi Group aim to address cystic fibrosis and pulmonary arterial hypertension, respectively.and with the Institute for Life Changing Medicines (ILCM) to develop an mRNA therapeutic for Crigler-Najjar Syndrome Type 1 (CN-1), an ultra-rare disease with only an estimated 70-100 known cases in the world.
AccessPrinciples.As we advance our pipeline, our commitment to working on multiple levels to optimize the impact of mRNA vaccines and therapeutics is reflected in our Access Principles:
Moderna is
We are committed to developing a broad portfolioadvancing clinical trial health equity by identifying the barriers that currently impede inclusion, and implementing approaches to more efficiently identify, engage, recruit and retain study participants from diverse backgrounds and vulnerable populations. When we recognized racial-ethnic minority under-representation in the Phase 3 clinical trial of vaccines and therapeutic solutions to address epidemiological challenges worldwide.
Moderna will invest in R&D in areas of unmet need.
Moderna will work to include communities that have historically been under-represented in clinical research inmRNA-1273, our development programs, as well as those that are disproportionately impacted by the respective diseases.
Moderna aims to provide effective and affordable vaccines and therapeutics to all populations.
Moderna will price its products through differential pricing frameworks.
Moderna is committed to participating in key public-private partnerships such as Gavi, the Vaccine Alliance.
Gavi-eligible countries will get Moderna’s lowest prices, and Moderna commits to an annual independent third-party audit on this commitment.
Looking ahead, we are actively considering the impact we can have with ouroriginal COVID-19 vaccine, as well aswe decided to slow down the overall clinical trial enrollment to ensure representative diversity. At the conclusion of enrollment, the clinical trial included more than 11,000 Participants of Color from diverse communities, representing 37% of the trial population. For the Phase 3 clinical trial of our other mRNA medicinescytomegalovirus (CMV) vaccine candidate, we achieved our goal of enrolling 42% Participants of Color in development, to help fight global pandemics and support public health planning.the US. Similarly, for the Phase 3 clinical trial of our RSV vaccine candidate, we again achieved our goal of enrolling at least 31% Participants of Color in the US.
moderna 2021 Proxy statement |23
Climate Change and the Environment
At Moderna, we are building a company that seeks to drive change through what we make and how we make it. With thisWe believe that ensuring the health of our planet is critical to positively impacting human health, and that it is our responsibility to grow Moderna in mind,a way that protects the planet and minimizes our impact on the environment. As an expanding company, we strivehave an opportunity to mitigate humangrow in a way that puts the protection of the environment as a key consideration in the design of new facilities, processes and products. As we continue to expand our manufacturing capabilities, we are committed to protecting the planet, based on a three-pronged strategy: sustainability by design, decarbonizing the value chain, and natural resource conservation.
We have set a goal of achieving net-zero carbon emissions for our Scope 1 and 2 emissions by 2030. We are also committed to defining a Scope 3 target that is in alignment with the Science-Based Targets Initiative.
Progress on climate and environmental initiatives in 2023 included the following actions:
| • | Enhanced our third-party assurance of energy and Scope 1 and Scope 2 emissions from Limited Assurance to Reasonable Assurance; |
| • | Published metrics related to Scope 3 emissions, water usage and waste management for our operations; |
| • | Incorporated sustainability into the design and construction of new manufacturing plants in Marlborough, Massachusetts; Laval, Canada; Melbourne, Australia; and Harwell, UK to reduce reliance on fossil fuels for thermal loads; |
| • | Completed a climate risk and scenario analysis project to better inform our understanding of climate-related risks and opportunities to our business; |
| • | Performed energy, water and waste assessments at our manufacturing facility in Norwood, Massachusetts to identify opportunities to reduce fossil fuel and water usage, improve operational efficiency and enhance our waste management program; and |
| • | Encouraged green transportation among our employees by offering fully subsidized public transport, bike sharing and free electric vehicle charging stations at our campuses. |
 | 2024 Proxy statement | 30 |
In February 2024, we opened our new Moderna Science Center in Cambridge, Massachusetts, a purpose-built space to support our next chapter of discovery. The high-performance building, which includes our principal executive offices, is targeting LEED Platinum Core & Shell and LEED Zero Carbon certifications. The building includes geothermal heating and cooling, rooftop solar, an exhaust air heat pump, and ultra-efficient building systems with acoustical and light pollution mitigation measures.
Further detail on key performance indicators and our efforts to minimize our impact on the environment whereare included in our ESG Report and on our website at modernatx.com under the “Responsibility—Corporate policies” section.
We recognize that our employees are key to our mission of delivering the greatest possible impact to people through mRNA medicines. As an organization, we are bold, collaborative, curious and pursue innovative ways to growrelentless. These values are underpinned by a core set of what we call “basecamp” values—integrity, quality, respect. Additionally, with the continued rapid growth of our business while minimizing our environmental footprint. We also aim to put Moderna at the forefront of managing the impact of waste from our business and to minimize the natural resourcesCompany, we use, while supporting employees’ efforts to do the same.
Our Cambridge headquarters is located in a LEED-certified building, andarticulated the Moderna Technology Center, locatedMindsets, which define how we behave, lead and make decisions. We believe our Mindsets are integral to our future success, and we continue to integrate them into every facet of how we identify, onboard, grow and manage our highest impact talent. New employees participate in Norwood, Massachusetts, wasour Moderna ONE onboarding program, which is an interactive, full-immersion learning experience designed to meet LEED certification criteria when it was first openedprovide the opportunity to engage with, better understand and learn how to apply the Mindsets in 2018. We also designed the facility to use 50 percent less water than comparable sites, and equipped it with electric vehicle charging stations and CO2 sensors to measure air quality.workplace.
In 2021, we are committing to sourcing these facilities with renewable energy, and to the extent these facilities are powered with sources other than renewable energy, we will be aiming to offset our carbon emissions by purchasing 100% certified renewable energy credits for every MWh consumed.
We will continue to explore ways to ensure that our operations promote long-term sustainability and that we minimize our footprint as we grow.
We had approximately 1,300 full-time employees as of December 31, 2020, representing a 59% increase over our 830 full-time employees as of the end of the prior year. This growth in our employee base has largely been as a result of developments related to our COVID-19 vaccine.
We have undertaken significant hiring to facilitate manufacturing of the vaccine, in addition to building out our commercial and regulatory organizations and other functions to support this roll-out. As part of this expansion, we also increased our hiring outside the United States during 2020, and at year-end we had employees in Switzerland, the United Kingdom, Canada and Spain. Much of this hiring has been of talent with experience at other pharmaceutical companies. We have also continued to hire talent to support our research and clinical capabilities across the rest of our pipeline, unrelated to our COVID-19 vaccine.
We operate in a highly competitive environment for human capital,talent, particularly as we seek to attract and retain talent with experience in the biotechnology and pharmaceutical sectors. Our workforce is highly educated, and as of December 31, 2020, 51%2023, 41% of our employees hold Ph.D., Doctorate, M.D., J.D., or Master’s degrees. Among our employees, 46%49% are female and 54% are male.female. Among our leadership (which we define as employees at the vice president level and above), as of December 31, 2020,2023, approximately 37%39% are female, an increase from 35% in 2019. 35%female. 45% of our U.S. employees identify as racially or ethnically diverse as of December 31, 2020,2023, an increase from 32%41% who identified as racially or ethnically diverse the prior year. In 2023, for the second year in a row, an outside statistical pay equity analysis confirmed zero statistically significant differences in pay across gender globally and across gender, race and ethnicity in the prior year.United States.
We had approximately 5,600 full-time employees as of December 31, 2023 in 19 markets around the world, with a presence in North America, Europe and the Asia-Pacific region. We are committed to building a culture of inclusion and belonging for all. In 2020,2023, we expandedcontinued to act on our emphasis oncommitment to belonging, inclusion &and diversity creating a Conscious Inclusion education series for managers, conducting several internal seminars, panels, and discussions on inclusion, launching a new employee resource group in support of our Black and African American employees, and adding a full-time senior-level role focused on belonging, equity, inclusion and diversity. In June 2020, our CEO also signed the CEO Action for Diversity and Inclusion Pledge, which commits Moderna to specific actions to cultivate a trusting environment where all ideas are welcomed—and people feel comfortable and empowered to have discussions about diversity and inclusion.by, among other things:
| • | continuing our monitoring and reporting of Company-wide gender and ethnicity data; |
| • | including a belonging, inclusion and diversity focus in every employee engagement survey; |
| • | continuing to invest in our Employee Resource Groups (ERGs), which are voluntary, employee-led groups that harness the power of belonging in service to our people, our Company and the community at large, and adding a ninth ERG, mCare, which is dedicated to supporting and empowering employees who are caregivers and parents; |
| • | gaining recognition from Disability:IN Inclusion and the American Association of People with Disabilities as a best place to work for disability inclusion; and |
| • | conducting diversity-related events, celebrations and learning opportunities for employees throughout the year. |
To help promote alignment between our employees and our shareholders, all employees participate in our equity programs through the receipt of new hire and annual equity grants,awards, and the percentage of equity as a component of overall pay mix increases with seniority. We believe that in addition to incentivizing growth that leads to shareholder value, broad eligibility for our equity programs further embeds our “We Act Like Owners” mindset and helps promote employee retention as these awards generally vest over a four-year period.
In response
 | 2024 Proxy statement | 31 |
Our employees are highly engaged, and our Company and team have been publicly recognized for our leadership, innovation and good corporate citizenship. Science magazine has ranked us as a top employer for each of the last nine years. Additionally, in 2023, Biospace ranked us the number one large employer in its 2024 Best Places to Work in Biopharma report for the COVID-19 pandemic, we undertook several initiativesthird consecutive year. We also received a perfect score from the Human Right Campaign’s Equality Index for 2023-2024. We measure employee engagement through a vendor-supplied engagement software, using validated external benchmarks to ensuretrack employee engagement factors.
We launched the safety of our workforceModerna Charitable Foundation in 2022 to support organizations and continuity of our operations.causes promoting public health and access to quality healthcare, advancing scientific education and innovation, supporting local and global communities, and advocating for inclusion and diversity. We createdaspire to make a Response Teamlasting impact in these focus areas through grant-making activity, by supporting relief efforts during humanitarian crises, and by supporting organizations that was responsible for implementing safety measures at our Cambridgematter to Moderna employees through the Foundation’s matching gifts program. During 2023, the Moderna Charitable Foundation made grants totaling approximately $7.2 million to local and Norwood sites. This included regular COVID-19 testing, temperature and health screeningglobal non-profit organizations working on causes aligned with the Foundation’s mission, as well as implementing digital tools$1.3 million in donations to facilitate contact tracingmatch employee giving. In 2023, the Foundation continued to provide significant support to two organizations seeking to improve access to healthcare in Africa—Seed Global Health and Amref. The Foundation has also committed to funding fellows focused on healthcare and advancing scientific education in partnership with Ashoka, a leading organization for social entrepreneurship.
We encourage individual employee volunteerism by providing personal protective equipment (PPE). Throughoutour people with paid time off to volunteer at the pandemic, much of our workforce has worked remotely, wherever possible. We also implemented remote hiring and onboarding programs to facilitate significant hiring during 2020 in a remote work environment. In December 2020, following the receipt of an Emergency Use Authorization from the FDA for our COVID-19 vaccine, we made the vaccine available to our employees and adult membersorganizations of their households to help ensure continuitychoice. During 2023, 70% of our operations due to the critical nature of our vaccine production.Moderna employees participated in tracked volunteering programs or employee giving, and volunteering hours increased 169% from hours reported in 2022.
moderna 2021 Proxy statement |24
 | 2024 Proxy statement | 32 |
Set forth below are the biographies for our current Executive Committee members. These individuals are critical to Moderna’s success and are responsible for leading our company to its next stage of development.

Age:
Joined Moderna and in current role since October 2011 | Stéphane Bancel, Chief Executive Officer |
| |
Before joining Moderna in October 2011, Mr. Bancel served for five years as Chief Executive Officer of the French diagnostics company bioMérieux SA. From July 2000 to March 2006, he served in various roles at Eli Lilly and Company, including as Managing Director, Belgium, and as Executive Director, Global Manufacturing Strategy and Supply Chain. Prior to Eli Lilly, Mr. Bancel served as Asia-Pacific Sales and Marketing Director for bioMérieux. He is currently a Venture Partner at Flagship | |
Education | |
•
• University of Minnesota, Master of Science in chemical engineering • Harvard Business School, M.B.A. |

Age:
Joined Moderna in
|
|
| |
Before joining Moderna in October 2022, Dr. Collins was employed by Novartis Education • University College Cork, Bachelor of
|
|
|
 Age:
Joined Moderna and in
|
Ms. Cronin oversees Moderna’s communications, branding and |
| |
Education • Smith College, B.A. in biology |
|
|
 Age:
Joined Moderna and in current role since October 2019 | Tracey Franklin, Chief Human Resources Officer |
| |
Before joining Moderna in October 2019, she was employed by Merck & Co. | |
Education | |
• Pennsylvania State University, B.A. in communication arts and sciences • Fairleigh Dickinson University, Masters in industrial and organizational psychology |
 | 2024 Proxy statement | 33 |
moderna 2021 Proxy statement |25
|
|
| |
| |
| |
|
 Age:
Joined Moderna in January 2013
| Stephen Hoge, M.D., President |
| |
Before joining Moderna in January 2013, he was employed by McKinsey & Company from 2005 to 2012, in roles of increasing responsibility, most recently as a Partner and a leader in the firm’s healthcare practice.
| |
Education | |
• Amherst College, B.A. in neuroscience • University of California, San Francisco, M.D. |

Age:
Joined Moderna and in current role since June |
Ms. Klinger oversees legal matters for Moderna, including corporate governance, intellectual property, litigation, compliance, privacy, as well as ESG strategy and global security. She is also the President of the Moderna Charitable Foundation. Before joining Moderna in June 2021, she was employed by Novartis from 2008 to 2021, most recently as Chief Legal Officer |
| |
| |
Education | |
•
•
|
moderna 2021 Proxy statement |26

Age:
Joined Moderna and in current role since January |
|
| |
Before joining Moderna in January 2023, Mr. Miller was employed by Capital One from 2021 to 2023 as Executive Vice President, Chief Technology Officer. He was employed by Mastercard from 2017 to 2021 as Executive Vice President, Operations and Technology; by Citi from 2015 to
Education • University of Waterloo, B.S. in • University of Nottingham, M.S. in human computer interaction in systems engineering |
|
|
 Age:
Joined Moderna and in current role since |
|
| |
Before joining Moderna in September 2022, he was employed by PerkinElmer from
| |
| |
|

|
|
| |
|
 | 2024 Proxy statement | 34 |
|
moderna 2021 Proxy statement |27
Our Board of Directors is committed to excellence in governance. Consistent with good governance practices and the requirements of the Dodd-Frank Act, shareholders have the opportunity to approve, on a non-binding, advisory basis, the compensation of Moderna’s named executive officers. This is commonly known as a “say on pay” proposal.
The say on pay proposal is not intended to address any specific item of compensation or the compensation of any particular officer, but rather the overall compensation of our named executive officers and our compensation philosophy, policies, and practices discussed in this proxy statement. The compensation of our named executive officers is disclosed in the Compensation Discussion and Analysis, the compensation tables, and the related narrative disclosure contained in this proxy statement. As discussed in these disclosures, we believe that our compensation policies and decisions are based on principles that reflect a “pay-for-performance” philosophy and are strongly aligned with our shareholders’ interests and consistent with current market practices. Our executive compensation program is designed to enable us to attract and retain talented and experienced executives to lead us successfully in a competitive environment.
We are asking our stockholdersshareholders to vote for the following resolution:
“RESOLVED, that the Company’s stockholdersshareholders approve, on a non-binding, advisory basis, the compensation of the Company’s named executive officers, as disclosed in this proxy statement, including the Compensation Discussion and Analysis, compensation tables and narrative discussion.”
Before you vote, we recommend that you read the Executive Compensation section that follows for complete information on our executive compensation programs and philosophy.
This vote is advisory, and therefore not binding on Moderna, the Board of Directors, or the Compensation and Talent Committee. However, our Board of Directors and Compensation and Talent Committee value your opinion and intend to consider the outcome of the vote when making compensation decisions in the future.
This non-binding, advisory proposal will be approved if it receives an affirmative vote of a majority of the shares of our common stock present in person or by proxy at the Annual Meeting and entitled to vote thereon.votes properly cast. If you ABSTAIN from voting on Proposal No. 2, the abstention will have no effect on the same effect as a vote AGAINST the proposal.results of this vote.
  | The Board of Directors recommends a vote “FOR” approval, on a non-binding, advisory basis, of the compensation of the Company’s Named Executive Officers. |
 | 2024 Proxy statement | 35 |
moderna 2021 Proxy statement |28
Dear Fellow Shareholders,
Fourteen years ago, Moderna’s founders set out to change the world by making mRNA medicines a reality for humanity. Moderna’s platform is delivering on the promise of mRNA as a result of significant investments since the Company’s founding. The COVID-19 pandemic challenged the Moderna team to deliver at an unprecedented pace, and the mRNA platform successfully enabled Moderna to develop and deliver one of the earliest and most effective vaccines against COVID-19. This success confirmed that our platform works, and we continue to aggressively expand our R&D capabilities across multiple areas. Moderna currently has 45 development programs, nine of which are in late-stage development, compared to 25 development programs at the end of 2020, when we only had one program in Phase 3.
2023 was a year of transition. The Company fell short of its COVID-19 vaccine product sales and operating income goals as we adapted to an endemic market, but the Company made significant progress in advancing its pipeline. The Moderna team pivoted to transform its COVID-19 business for the endemic setting in 2023 by resizing its manufacturing footprint, positioning the COVID-19 franchise for profitability in 2024 and beyond. Moderna continues to enhance its commercial capability while building out systems and processes to ensure operational excellence throughout the organization. With these operating improvements, and continued pipeline success, we hope to lay the groundwork for future growth.
A key differentiator at Moderna has always been its people and their willingness to innovate and drive solutions. Our executive compensation program continues to be centered on at-risk, performance-based incentives, ensuring that Moderna’s executive compensation is linked to both the Company’s financial results and execution of our long-term strategic objectives, which ultimately drive the creation of long-term shareholder value. We have highlighted below key elements of the executive compensation framework and how they tie to both our current and future expectations of performance for the Company and leadership team.
2023 Corporate Performance — Executive Team Bonuses Paid at 81% of Target
| • | Moderna fell short in certain key areas for 2023 performance, as discussed in the following pages. As a result, the CEO and Executive Committee bonuses were calculated by multiplying the 90% corporate funding by a 90% individual performance factor, which resulted in a final 2023 bonus payout of 81% of target. This approach acknowledges that while each member of the Executive Committee made meaningful contributions to our Company’s progress during 2023, the Company’s success in achieving its objectives requires the Executive Committee to execute and deliver operationally as a team. Further details on corporate performance are included in the “2023 Corporate Objectives” section on page 48. |
2021-2023 PSU Program Update — 200% Performance Achievement
| • | The 2021-2023 PSU program resulted in a maximum payout. 2021 was the first year we included PSUs in Moderna’s equity program, and we set ambitious regulatory and clinical development goals that the Committee agreed would represent significant over-achievement in terms of obtaining our first full product approvals in a number of major markets and significantly advancing and diversifying our portfolio beyond our COVID-19 vaccine. |
| • | While the vesting percentage was 200% of target, the value that vested was 129% of the grant value as a result of the stock price decline over the three-year performance period ($132.19 to $85.37). |
2023 Stock Price Impact on Value of Equity Awards
| • | Equity awards are the largest component of our executive compensation program, representing no less than 70% of the target total compensation for each of our executives. Therefore, stock price has a significant impact on the compensation realized by our executives, thus aligning our executive and shareholder experiences. The CEO and average other NEO outstanding equity award values as of December 31, 2023 decrease by 51% and 49%, respectively, when the awards are compared using the stock price from the previous year end ($179.62 vs. $99.45, a 45% decrease). All stock options granted from 2021 through 2023 were underwater as of December 31, 2023, with exercise prices for those options significantly above prevailing stock prices. |
In the fall of 2023, we engaged with shareholders to discuss Moderna’s performance and compensation framework. Based on the valuable feedback we have received from you, our shareholders, the Compensation and Talent Committee has taken deliberate actions regarding our executive compensation program to reinforce our pay-for-performance philosophy and ensure alignment with our disciplined investment strategy, as outlined below.
 | 2024 Proxy statement | 36 |
Increased PSU Weighting for 2024 Long-Term Incentive Awards
| • | Our CEO’s 2024 annual equity award is comprised of 50% of PSUs (increase from 25%) and 50% stock options (decrease from 75%). |
| • | Other NEOs and Executive Committee members have an equal mix of PSUs, stock options and RSUs for their annual equity awards, beginning in 2024 (PSU increase from 25% to 33 1/3%). |
| • | This shift further ties executive compensation to the achievement of key pipeline advancement and financial objectives, while achieving a balance in terms of maintaining stock options, which only deliver value with stock price growth. |
Added Long-Term Financial Metrics to our 2024 – 2026 PSU Program
| • | Our 2024-2026 PSUs include long-term financial metrics for the first time, reflecting Moderna’s continued evolution as a commercial company. |
| • | The financial metrics reflect the key goals that management has communicated and incentivizes achieving those goals by the end of 2026. |
| • | The program also continues to remain focused on expanding and diversifying our portfolio into non-respiratory therapeutic areas to drive long-term shareholder value. |
Enhanced CD&A Disclosure
| • | We have augmented our CD&A to provide shareholders with a clearer understanding of our short-term and long-term incentive programs and the strong performance necessary to achieve our financial and strategic objectives. |
| • | These changes are part of our ongoing efforts to demonstrate the direct linkage between compensation and company performance. |
In 2024, Moderna is focused on executing its commercial strategy, investing with discipline, and advancing its late-stage pipeline to drive organic sales growth. We have the utmost confidence in our Executive Committee to lead the organization, execute on the Company’s strategy, and deliver on these ambitious goals.
We are grateful for your support as we continue to navigate the exciting opportunities and challenges ahead. Your feedback is invaluable to us, and we remain dedicated to open communication with our shareholders.
On behalf of the entire Committee, we thank you for your partnership as we strive to bring revolutionary mRNA medicines to patients and create value for all our shareholders.
Sincerely,
François Nader
Chair, Compensation and Talent Committee
 | 2024 Proxy statement | 37 |
Compensation Discussion and Analysis
This Compensation Discussion and Analysis describes our executive compensation program and the 20202023 compensation for our named executive officers (our “NEOs”)NEOs). This Compensation Discussion &and Analysis should be read with the compensation tables and related disclosures for our NEOs.
Table of Contents | |
| Executive Summary |
Moderna is advancing messenger RNA (“mRNA”) science(mRNA) therapeutics and vaccines to create a new class of transformative medicines for patients.deliver the greatest possible impact to people through mRNA medicines are designed to direct the body’s cells to produce intracellular, membrane or secreted proteins that can have a therapeutic or preventive benefit and have the potential to address a broad spectrum of diseases.medicines. Our mRNA platform builds on continuous advances in basic and applied mRNA science, delivery technology and manufacturing, providing the Company the capability to pursue in parallel a robust pipeline of new development candidates. We are developing therapeutics and vaccines for infectious diseases, immuno-oncology, rare diseases cardiovascular diseases, and autoimmune and inflammatory diseases, independently and with strategic collaborators.
In 2020, we commercialized our first product,
2023 was a year of transition for the Moderna COVID-19 Vaccine (also referred to as mRNA-1273). We developed and brought this highly-effective vaccine to market on an unprecedented timeframe, demonstrating the incredible potentialCompany, marked by a number of mRNA science. We currently have 24 mRNA development programs in our portfolio across six separate modalities, with 13 programs having entered the clinic.
Due to our progress over the last year, Moderna is entering 2021 as a commercial company, with a strong cash position, clear strategic priorities and a team poised to continue advancing mRNA vaccines and therapeutics into new areas of high unmet need. Our strategic priorities heading into 2021 are clear:
Maximize the impact of Moderna COVID-19 Vaccine access and the value creation of this product between now and the end of 2021.
Accelerate vaccine development to advance our pipeline and bring new vaccines to market.
Generate human proof-of-concept data in autoimmune diseases, cardiovascular diseases, oncology and rare diseases.
Continue to expand the use of mRNA technology to maximize the potential impact we can have on patients.
Our executive compensation programs have been designed with the goal of focusing our team on delivering on the promise of mRNA medicine, while maintaining enough flexibility to adaptchallenges in the short-term,COVID-19 vaccine market that caused us to fall short on our financial goals, but with considerable progress as we advanced several other programs into late-stage development. Highlights included:
| • | We achieved 48% retail U.S. market share for the 2023 COVID-19 vaccine fall season (compared to 37% in the 2022 fall season), demonstrating our ability to compete in a commercial market. |
| • | We achieved significant progress advancing nine programs across our respiratory, latent, oncology and rare disease franchises into late-stage development. |
| • | We pivoted to significantly resize our manufacturing infrastructure to reflect future anticipated market demand for our COVID-19 franchise. |
During 2023, we made significant progress advancing our mRNA platform and our clinical pipeline, but from a financial perspective, our corporate performance did not meet our expectations as we fell short of our objectives in 2020.terms of product sales and operating income. The corporate objectives for 2023 were:
| 1. | Execute the operational and sales plan for COVID-19 vaccine. |
| 2. | Build an unrivaled seasonal respiratory vaccine franchise. |
| 3. | Execute on a bold campaign of cancer vaccine studies. |
| 4. | Advance rare metabolic disease programs. |
| 5. | Drive rapid advancement and growth in our latent vaccine portfolio. |
| 6. | Deliver the next-generation pipeline and platform. |
| 7. | Build a culture of learning, and strengthen our processes and digital systems. |
In designing our annual corporate objectives, we focus on those shorter-term milestones that we believe will help us move closer to bringing new mRNA medicines to patients. But weOur Executive Committee members are also want our team to focus on the long-term output from these efforts, which is why in addition to our traditional equity programs, which are primarily weighted toward stock options, with a portion granted to executives other than the CEO in RSUs, we are introducing performance-based restricted stock units (“PSUs”) as part of our compensation program for our Executive Committee in 2021. These PSUs will have a(PSUs) with three-year performance period andperiods that maintain focus on our long-term pipeline goals.strategy. Stock options help reinforce the long-term orientation of our executives as they have a four-year vesting period and ten-year exercise period.expire after ten years, and only convey value if our stock price appreciates. We believe this general design, which focuses on long-term objectives, coupled with an ability to pivot quickly to adapt our compensation plans, has enabled us to deliver for patients and our shareholders.
 | 2024 Proxy statement | 38 |
Our executive compensation program seeks to incentivize and reward strong corporate performance. Highlights of our 20202023 corporate performance are set forth below.
| • | The most heavily weighted factor in the assessment of our 2023 performance is product sales for our COVID-19 vaccine. While we achieved net product sales of $6.7 billion for the year, this performance fell short of our internal targets due to lower demand as we entered the endemic market. |
| • | In terms of operating income, our operating loss of $4.2 billion for 2023 also fell significantly short of internal targets, with performance below threshold, resulting in no payout for this part of the scorecard. |
Developed a highly-effective vaccine against COVID-19, progressing from sequence selection, through clinical trials (including a 30,000 participant Phase 3 clinical trial), to Emergency Use Authorization from the FDA in just over 11 months—followed shortly thereafter by authorizations in several other jurisdictions.
moderna 2021 Proxy statement |29
BackBy contrast, we made significant strides in continuing to Contents
Expanded manufacturing capacity at our Norwood, Massachusetts plant and partnered with other companies in the U.S. and abroad to facilitate manufacturing of between 700 million and 1 billion doses of our COVID-19 vaccine in 2021 (enough to vaccinate 350 million to 500 million people), delivering approximately 17 million doses in 2020.
Began building out a commercial organization and signed advanced purchase agreements for approximately 520 million doses of COVID-19 vaccine in 2021 (as of December 31, 2020), for total of $11.7 billion, including 200 million doses to the U.S. government. In February 2021, the U.S. government agreed to purchase an additional 100 million doses, for 300 million doses in total.
Despite the pandemic, we continued to make significant progress across the pipeline, including in the following areas:
Generated human proof of concept data for multiple medicines, including achieving proof of concept (immunogenicity) in the Phase 2 trial of our COVID-19 vaccine and efficacy in the Phase 3 trial, as well as achieving proof of concept in the Phase 2 trial for our cytomegalovirus (“CMV”) vaccine (mRNA-1647).
Executed on our current development pipeline, delivering on nearly all core operating objectives for clinical lot manufacture and delivery and adding 11 new programs, in addition to our COVID-19 vaccine.
Nominated seven new development candidates by the end of 2020—well in excess of our goal of three new candidates—including our COVID-19 vaccine (mRNA-1273), our next generation COVID-19 vaccine (mRNA-1283), an Epstein-Barr Virus vaccine (mRNA-1189), influenza vaccines (mRNA-1010, mRNA-1020 and mRNA-1030), pediatric RSV vaccine (mRNA-1345), PD-L1 (mRNA-6981) for autoimmune hepatitis, and IL-2 (mRNA-6231) for autoimmune disorders.
Made substantial progress in one new modality, for pulmonary delivery, with evidence of nebulized transfection in vivo pulmonary delivery and in vitro pulmonary delivery.
Ended 2020 with cash, cash equivalents and investments of approximately $5.25 billion, a significant increase over $1.26 billion at the end of 2019, providing us significant resources to expandadvance our pipeline, and development programs.including:
Net cash provided by operating activities in 2020, less purchases of property and equipment, was $1.96 billion. This reflected a net loss of $747.1 million, plus non-cash adjustments of $197.0 million, a net change in assets and liabilities of $2.58 billion and purchases of property and equipment of $67.4 million. Net cash provided by operating activities was significantly better than original consensus estimates of a loss, which anticipated net cash used for operating activities and purchases of property and equipment of $498 million. The difference was driven primarily by customer deposits for supply of our COVID-19 vaccine. Third quarter 2020 marked the first cash flow positive quarter for the company.
Recognized year-over-year stock price appreciation of 434% (from closing price of $19.56 on December 31, 2019 to a closing price of $104.47 on December 31, 2020), which is in the top quartile of our peer group and for the NASDAQ Biotechnology Index over the same period in terms of total shareholder return.
moderna 2021 Proxy statement |30
| • | Received two U.S. FDA supplemental Biologics License Application (BLA) approvals for our updated COVID-19 vaccines; |
| • | Filed for the BLA for our RSV vaccine with the FDA; |
| • | Initiated a Phase 3 trial of our combination vaccine against seasonal flu and COVID-19; |
| • | Advanced our latent vaccine pipeline by fully enrolling participants in the cytomegalovirus (CMV) pivotal Phase 3 study to evaluate its efficacy, safety and immunogenicity; |
| • | Identified a dose for expansion studies for our propionic acidemia therapeutic, and met our enrollment targets for our rare disease programs; and |
| • | Demonstrated the clinical benefit of our INT program in melanoma, showing significant and clinically meaningful improvement in recurrence-free survival and reducing the risk of recurrence or death by 49% versus KEYTRUDA alone. |
Below is a summary of our named executive officers (“NEOs”)NEOs for 20202023 and a brief overview of the executive compensation actions that were taken for each of these NEOs. The individuals set forth below represent all of our executive officers during 2023 for purposes of Rule 3b-7 of the Securities Exchange Act of 1934, as amended.
In response to the challenges Moderna faced in meeting our corporate objectives for 2023, particularly with respect to meeting our financial goals, the Compensation Committee decided to provide uniform bonuses to the CEO and other members of the Executive Committee (including all of our NEOs), establishing collective accountability for the Company’s overall performance. Each member of the Executive Committee received an individual multiplier of 90%, which, when combined with the 90% corporate multiplier, resulted in bonus payments at 81% of target. This approach acknowledges that while each member of the Executive Committee made meaningful contributions to our Company’s progress during 2023, the Company’s success in achieving its objectives requires the Executive Committee to execute and deliver operationally as a team.
Chief Executive | Stéphane Bancel | ||
| Compensation for Mr. Bancel for 2023 was as set forth below. | |||
 | Salary: $1,575,000 (5% increase over 2022) Bonus: $1,913,625, based on target of 150% of salary, with 81% payout, reflecting 90% individual assessment, as described above. Equity Awards(1): $15,000,000, delivered 75% in stock options and 25% in PSUs (based on 2022 performance) | ||
| 1) | Amounts shown for equity awards reflect target value, not grant date fair value. For grant date fair value, see the Summary Compensation Table on page 59. |
 | 2024 Proxy statement | 39 |
 |

Chief Age: 47 | |||
| Compensation for Mr. Mock for 2023 was as set forth below. | |||
 | Salary: $800,000 (6.7% increase over 2022) Bonus: $583,200, based on target of 90% of salary, with 81% payout, reflecting 90% individual assessment, as described above. Equity Awards (1): $3,500,000, delivered 50% in stock options, 25% in RSUs and 25% in PSUs (based on 2022 performance) | ||
 President Age: 48
|
| Stephen Hoge, M.D. | |
| Compensation for
| ||
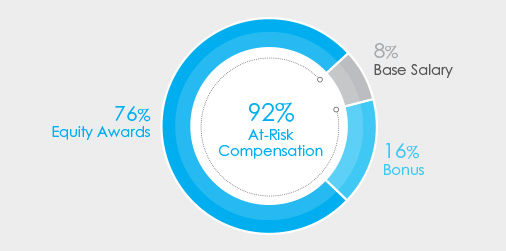 |  | Salary: $ Bonus: $ Equity $ | |

Former Chief
|
| ||
| |||
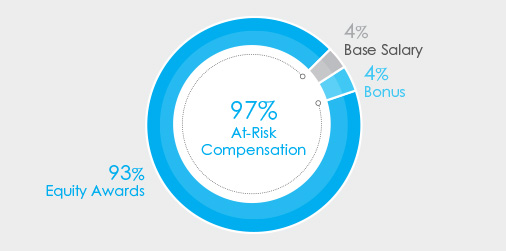 |
| ||

Age: 45
|
| Arpa Garay | |
| Compensation for
| ||
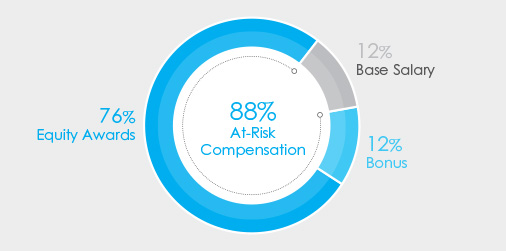 |  | Salary: $ Bonus: $ Equity $ | |
Chief Legal Officer and Corporate Secretary | Shannon Thyme Klinger | ||
moderna 2021 Proxy statement |31

|
| |
| ||
  | Salary: $ Bonus: $ Equity $ | |
| 1) | Amounts shown for equity awards reflect target value, not grant date fair value. For grant date fair value, see the Summary Compensation Table on page 59. | |

|
 |
| 40 |
| ||
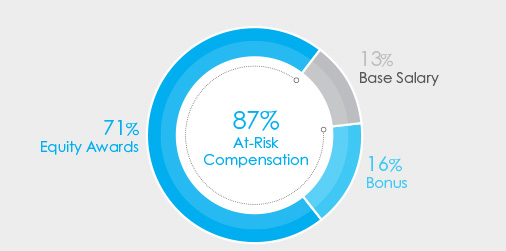 |
| |

|
| |
| ||
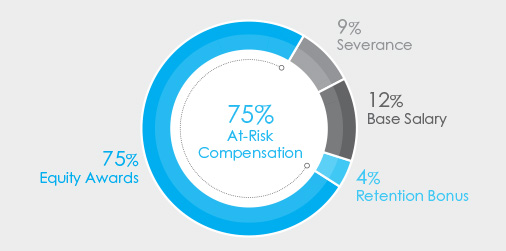 |
| |
moderna 2021 Proxy statement |32
Overview of Executive Compensation Program
Executive Compensation Philosophy
Our executive compensation program is guided by our overarching philosophy of paying for demonstrable performance.performance and impact. We are focused on our mission to “Deliver on the promise ofgreatest possible impact to people through mRNA science to create a new generation of transformative medicines for patients.medicines.”
During 2020, we made incredible and tangible progress on this decade-long journey, as demonstrated by our development of a highly-effective vaccine using mRNA technology to combat the COVID-19 pandemic on an unprecedented timeline.
We believe that our compensation philosophy helps align our team around executing on our mission, of delivering for patients, which ultimately leads to greater shareholder value. All full-time employees at Moderna, regardless of their level, receive equity as part of their compensation, aligning them to investors and making them personally invested in our mission. As we continue delivering on the promise of mRNA science, we recognize that our executive compensation programs must also continue to attract and retain a talented team who can help us achieve this mission.
Consistent with this philosophy, we have designed our executive compensation program to achieve the following primary goals:
| • | Pay for performance by establishing competitive opportunities to incentivize high performance and deliver greater rewards when corporate and individual performance exceed expectations and lower compensation when corporate or individual performance falls short. Performance is measured by financial, operating and strategic performance, return to shareholders and individual contributions. |
| • | Attract, motivate and retain industry-leading talented individuals through well-designed compensation programs that motivate our executives to achieve rigorous corporate objectives that are important to our business and long-term success. |
| • | Establish a competitive rewards program by evaluating the practices of our peers and market data to validate that we are competitive with other companies who compete with us for talent. |
| • | Align the interests of our executives and our shareholders to drive the creation of sustainable long-term value. |
| • | Balance a combination of compensation elements to achieve an appropriate balance between cash and equity awards. The annual cash bonus is intended to motivate individuals to successfully execute on short-term financial and strategic objectives. Equity awards are intended to focus executives on the long-term success of the organization. |
Pay for performance is a foundational element within Moderna’s compensation program structure. We compare CEO total realizable pay (as defined below) to stock price performance for Moderna and its peers. Our peer group represents companies of comparable size and business as outlined by the criteria on page 45.
We looked at pay and performance alignment over two periods as follows:
| • | 3-Year Analysis (2021-2023): The Company’s total shareholder return (TSR) performance over the three-year period ending December 31, 2023 was at the 36th percentile among these peer companies and the total realizable pay for our CEO over the same period was at the 4th percentile. |
| • | 5-Year Analysis (2019-2023): The Company’s TSR performance over the five-year period ending December 31, 2023 was at the 100th percentile among these peer companies and the total realizable pay for our CEO over the same period was at the 72nd percentile. |
The three-year analysis shows that realizable pay and TSR performance were in the bottom left quadrant due to lagging share price performance. In comparison, over five years, realizable pay and TSR performance were in the top right quadrant due to leading stock price performance. Both analyses demonstrate our strong pay for performance alignment as the Company’s relative stock price performance ranked higher than CEO relative pay.
| CEO Realizable TDC Rank vs. 3-Year TSR Performance Rank | CEO Realizable TDC Rank vs. 5-Year TSR Performance Rank |
 |  |
 | 2024 Proxy statement | 41 |
attract, motivateRealizable Total Direct Compensation (Realizable TDC) is defined as the sum of the following components: actual base salaries, short-term incentive awards, and retain top-performing senior executives;
establish compensation opportunities thatlong-term incentive awards paid over the preceding three- and five-year periods. The value of all in-the-money stock options granted during the preceding three- and five-year period and RSUs and PSUs granted over the preceding three- and five-year periods (reflecting actual performance results or estimated performance based on peer proxy disclosures) are competitivevalued as of December 31, 2023.
TSRs are calculated based on the dividend adjusted closing price per share of common stock on the applicable exchange from January 1, 2021 through December 31, 2023 and reward performance;from January 1, 2019 through December 31, 2023 for the respective periods.
align the interests of our senior executives with the interests of our stockholders to drive the creation of sustainable long-term value.
In 2020, 96%2023, 88% of stockholdersshareholders who voted at our Annual Meeting who voted on our “say on pay” proposal supported the pay actions we took in the prior year. We believe this vote is indicative ofsignals strong overall support for our pay programs and their general design. Accordingly,Based upon feedback from our investors since the 2023 Annual Meeting, our Compensation Committee maintainedhas made the general structure of our pay programs for 2020.following enhancements:
| • | increased the weighting in our long-term incentive program for performance-based restricted stock units (PSUs) for our executive team beginning in 2024, and |
| • | continued to provide greater visibility into those goals that will inform payouts on those PSU awards and for the goals in our corporate scorecard. For additional detail, see “Letter from the Compensation and Talent Committee,” on page 36, and “Governance—Shareholder Engagement” on page 20. |
Our executive compensation program is designed to be reasonable and competitive, and balance our goal of attracting, motivating, rewarding and retaining top-performing senior executives with our goal of aligning their interests with those of our stockholders.shareholders. Our compensation and talent committee of our board of directors, or Compensation Committee annually evaluates our executive compensation program to ensure that it is consistent with our short-term and long-term goals and the dynamic nature of our business.
Our executive compensation program consists of a mix of compensation elements that balance achievement of our short-term goals with our long-term performance. We provide short-term incentive compensation opportunities in the form of annual cash bonuses, which focus on our achievement of annual corporate goals. We also provide long-term incentive compensation opportunities in the form of equity awards, which, have historicallyuntil recent years, consisted primarily of stock options. Due to Moderna’s pre-commercial stage of development until 2020, our equity programs were primarily focused on stock options to provide our team with a strong incentive to pursue growth that would result in stock price appreciation over the long-term.
As we havethe Company has matured, as a company, our equity programs have also evolved. In 2020,2021, we introduced a limited amount of equity choice for our employees, including our executive team members (other than our CEO), which allowed executives to receive up to 25% of their annual equity grant in the form of Restricted Stock Units (“RSUs”). This program was designed to provide employees (other than our CEO) with increased flexibility and to tailor their equity awards to their particular risk tolerance and to further promote employee engagement.
Stock options granted to our executive team in 2020 continued to have a standard vesting schedule of 25% after one year and quarterly vesting over the next three years. RSUs granted to the executive team were subject to a longer “cliff” vesting period of 50% on the second anniversary of the grant date and quarterly over the next two years. We believe RSUs also reward growth in the market price of our common stock because they derive additional value from stock price appreciation, they help our executives build actual stock ownership, and they are less dilutive to our stockholders because they require fewer shares than stock options to deliver the same dollar value of an award. In addition, we believe that the multi-year vesting requirements applicable to both stock options and RSUs encourage retention because our senior executives are incentivized to remain employed through the vesting period.
Beginning in 2021, we are also introducing performance-based restricted stock units (PSUs)PSUs into our equity programs for our executives. TheseExecutive Committee members. As further described on page 53 below, these PSUs focusfocused on achieving those goals that the achievementCompensation Committee recognized would be key to our long-term success, particularly once COVID-19 vaccine revenues peaked.
Early in 2021, Moderna had still not received full approval for any product—just an emergency use authorization for its COVID-19 vaccine—and the COVID-19 vaccine represented our only product to have reached a Phase 3 trial. Therefore, the goals for 2021-2023 reflected the importance of pipeline(i) obtaining full approval for our COVID-19 vaccine in key markets, (ii) commencing Phase 3 or registrational studies for non-COVID programs, and (iii) advancing our non-vaccine, therapeutic programs to positive Phase 1/2 early clinical readouts. Each of these goals for this first class of PSUs was achieved above target. The goals for our development programs over a three-year performance period, with “cliff” vesting at the endother classes of the period only if the pipeline goalsPSUs are achieved. The objective of this program is to promote the achievement of longer-term objectives and the development of a robust pipeline of mRNA medicines. In this first year,further described on page 54 below. For the PSUs represent 25% ofgranted in 2021, 2022 and 2023, the overall grant date fair value of the equity package awarded tolong-term incentive mix was prescribed for each of our Executive Committee members. Formembers as described below. Beginning in 2024, PSUs will represent 50% of the weighting for equity awards to our CEO, the remaining 75% of his 2021 equity award was granted in stock options, and for the restone-third of the weighting for equity awards to our other Executive Committee the remainder of the annual award consisted of 50% options and 25% RSUs. members.
| 2023 | 2024 | ||||||
| Equity Mix | Stock Options | PSUs | RSUs | Stock Options | PSUs | RSUs | |
| CEO | 75% | 25% | — | 50% | 50% | — | |
| Other Executive Committee Members | 50% | 25% | 25% | 33% | 33% | 33% | |
 | 2024 Proxy statement | 42 |
Our executive compensation program is also designed to incorporate sound practices for compensation governance. Below we summarize such practices.governance as summarized below.
moderna 2021 Proxy statement |33
WHAT WE DO |
 |
| |||
 | MaintainanIndependentCompensationCommittee. The Compensation Committee consists solely of independent directors. |  |
| |
  | RetainanIndependentCompensationAdvisor. The Compensation Committee engages its own advisor to provide information, |  |
| |
  |
|  |
| |
  |
|  |
| |
  | Use a Pay-for-Performance Philosophy. | |||
 | Require 10b5-1 Plans. Require our executives to plan sales of Moderna stock in advance through the use of 10b5-1 plans. | |||
 |  | |||
 | Maintain a Clawback Policy. We have a clawback policy applicable to performance-based compensation for our Executive Committee, which would apply in the event of a financial restatement or other improper conduct causing material financial, operational or reputational harm. | |||
 | Mitigate Undue Risk-Taking. Employ the use of multiple performance goals and performing annual compensation risk assessments. | |||
| WHAT WE DON’T DO | ||||
 | No Special Health and Welfare Benefits. Our executive officers participate in our health and welfare benefits programs on the same basis as our other employees, other than eligibility for additional long-term disability insurance that we offer at the VP level and up. | |||
 | No Executive Retirement Plans. We do not offer pension or retirement plans to our executive officers that are different from or in addition to those offered to our other employees. | |||
 | No Post-Employment Tax Payment Reimbursement. We do not provide any tax reimbursement payments (including “gross-ups”) on any change-in-control or severance payments or benefits. | |||
 | No Hedging or Pledging Our Equity Securities. We prohibit our executive officers, the members of our Board and certain other employees from hedging or pledging our securities. | |||
 | No StockOptionRe-PricingunderCurrentStockPlan. Our 2018 Stock Plan does not permit stock options to be repriced to a lower exercise or strike price without the approval of our | |||
 |
| |||
 | 2024 Proxy statement | 43 |
 |
| |||
 |
| |||
Role of the Compensation Committee and the Board
of Directors
The Compensation Committee, which is comprised entirely of independent directors, is responsible for discharging our Board of Directors’ responsibilities relating to compensation of our directors and executives, overseeing our overall compensation structure, policies and programs, and reviewing our processes and procedures for the consideration and determination of director and executive compensation. The primary objective of the Compensation Committee is to develop and implement compensation policies and plans to attract and retain key management personnel, motivate management to achieve our corporate goals and strategies, and align the interests of management with the long-term interests of our stockholders.shareholders. We have not adopted formal guidelines for allocating total compensation between long-term and short-term compensation, cash compensation and non-cash compensation, or among different forms of non-cash compensation.
Our Compensation Committee has engaged Pay Governance LLC, an independent executive compensation consultant, to provide guidance with respect to the development and implementation of our compensation programs.
Pursuant to our Equity Award Grant Policy, the Compensation Committee has delegated to our CEO and our Chief Human Resources Officer (CHRO) the authority to approve grants of equity awards, subject to certain parameters, under the 2018 Stock Plan. See “Other Compensation Policies and Practices—Equity Award Grant Policy.”
moderna 2021 Proxy statement |34
The Compensation Committee reviews and approves the primary elements of compensation—base salary increases, annual cash bonuses, and annual equity awards—for our NEOs (other than our CEO), as authorized by the Board of Directors pursuant to the Compensation Committee Charter.charter. Our Board of Directors reviews and provides final approval for the primary elements of compensation awarded to our CEO after recommendation by the Compensation Committee.
When reviewing and approving, or recommending to the Board of Directors, as applicable, the amount of each compensation element and the target total compensation opportunity for our executive officers, the Compensation Committee considers the following factors:
our performance during the year, based on business and corporate goals and priorities established by the CEO and the Board of Directors;
| • | our performance during the year, based on business and corporate goals and priorities established by the CEO and the Board; |
| • | each executive officer’s skills, experience and qualifications relative to other similarly situated executives at the companies in our compensation peer group; |
| • | the scope of each executive officer’s role compared to other similarly situated executives at the companies in our peer group; |
| • | the performance of each individual executive officer, based on an assessment of his or her contributions to our overall performance, ability to lead his or her department and work as part of a team, all of which reflect our values; |
| • | compensation parity among our executive officers; |
| • | our retention goals; |
| • | the compensation practices of our peer group; and |
| • | our CEO’s recommendations with respect to the compensation of our other executive officers. |
each executive officer’s skills, experience and qualifications relative to other similarly-situated executives at the companies in our compensation peer group;
the scope of each executive officer’s role compared to other similarly-situated executives at the companies in our compensation peer group;
the performance of each individual executive officer, based on an assessment of his or her contributions to our overall performance, ability to lead his or her department and work as part of a team, all of which reflect our values;
compensation parity among our executive officers;
our retention goals;
the compensation practices of our compensation peer group; and
our CEO’s recommendations with respect to the compensation of our other executive officers.
These factors provide the framework for compensation decisions for each of our executive officers, including our NEOs. The Compensation Committee and the Board of Directors, as applicable, do not assign relative weights or rankings to these factors, and do not consider any single factor as determinative in the compensation of our executive officers. Rather, as we operate in a rapidly changing industry and most of our programs are still in clinical or preclinical stage, the Compensation Committee and the Board of Directors, as applicable, believes it is best to rely on their own knowledge of our business and industry and therefore they use judgment in assessing these factors and making compensation decisions.
In discharging its responsibilities, the Compensation Committee works with management, including our CEO. Our management assists the Compensation Committee by providing information on corporate and individual performance, market compensation data and management’s perspective on compensation matters.
In addition, at the beginning of each year, our CEO generally reviews the performance of our other executive officers, including our other NEOs, based on our achievement of our corporate goals and each executive officer’s achievement of his or her departmental and individual goals established for the prior year and his or her overall performance during that year. The Compensation Committee solicits and reviews our CEO’s recommendations for base salary increases, annual cash bonuses, annual equity awards and any other compensation opportunities for our other executive officers, including our other NEOs, and considers our CEO’s recommendations in determining such compensation.
The Compensation Committee engages an external compensation consultant to assist it by providing information, analysis and other advice relating to our executive compensation program. For 2020,2023, the Compensation Committee engaged Pay Governance as its compensation consultant to advise on executive compensation matters including:
| • | review and analysis of the compensation for our executive officers, including our NEOs, and our Board of Directors; |
| • | assistance on incentive program design and discussion on executive compensation and governance trends; |
 | 2024 Proxy statement | 44 |
review and analysis of the compensation for our executive officers, including our NEOs, and our Board of Directors;
| • | review and input on the Executive Compensation section of our Proxy Statement for our 2024 Annual Meeting of Shareholders; |
| • | research, development and review of our compensation peer group; and |
| • | support on other compensation matters as requested throughout the year. |
assistance on incentive program design and discussion on executive compensation and governance trends;
review and input on the Executive Compensation section of our Proxy Statement for our 2021 Annual Meeting of Stockholders;
research, development and review of our compensation peer group; and
support on other compensation matters as requested throughout the year.
Pay Governance reports directly to the Compensation Committee and to the Compensation Committee chairman. Pay Governance also coordinates with our management for data collection and job matching for our executive officers. Our Compensation Committee charter requires that our compensation consultant is independent of Company management. During 2020,2023, Pay Governance did not provide services to us other than the services to our Compensation Committee described herein. Our Compensation Committee performs an annual assessment of its compensation consultants’consultant’s independence to determine whether the
moderna 2021 Proxy statement |35
consultants are consultant is independent and in 20202023 has determined that Pay Governance is independent pursuant to the Nasdaq listing standards of the relevant Nasdaq and SEC rules and has determined that no conflict of interest has arisen as a result of the work performed.
For purposes of comparing our executive compensation against the competitive market, the Compensation Committee reviews and considers the compensation levels and practices of a group of peer companies. This compensation peer group consists of public biotechnology and pharmaceutical companies against which we may compete for talent and that are similar to us in termsacross a number of factors, including market capitalization, stage of development, geographical location and number of employees. The Compensation Committee reviews our compensation peer group at least annually and makes adjustments to our peer group ifas necessary, taking into account changes in both our business and our peer companies’ businesses. The Compensation Committee also uses market data from our compensation peer group and from the Radford Global Life Sciences Compensation survey as one factor in evaluating whether the compensation for our executive officers is competitive in the market. The Compensation Committee and the Board of Directors, as applicable, also rely on their own knowledge and judgment in evaluating market data and making compensation decisions.
Since becoming a public company in 2018, our Compensation Committee has used our Peer Grouppeer group to assist in assessing annual base salary, target bonus and equity awards for our NEOs and other senior level employees. To determine the composition of the Peer Grouppeer group for 2020,2023 the Compensation Committee considered the following criteria:
publicly-traded companies listed in the United States;
companies in the biotechnology sector;
similar market capitalization-within a range of approximately 0.4x to approximately 3.3x our then-current market capitalization;
the stage of development of each company’s development candidates;
companies developing products in our target areas of infectious disease vaccines, rare diseases and immune-oncology; and
companies with employee populations reflecting similar organization scale to Moderna.
This analysis led to the initial selection of the following peer group which was used to make the relevant compensation assessments for 2020.
| • | commercial stage pharmaceutical and biotechnology companies with global revenues, double-digit pipelines and multiple late-stage candidates; |
| • | market capitalization, generally selecting companies with a market capitalization around Moderna’s peer median; |
| • | companies with significant commercial revenues (generally more than $2 billion annually); |
| • | headcount factoring in Moderna’s growing headcount as company grows globally and commercializes; and |
| • | R&D expenditure to provide context for scale of R&D organization and to balance revenue perspective as focus shifts to commercial revenue. |
 | 2024 Proxy statement | 45 |
| ||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Based on a review ofthese factors, the compensation peer group set forth below was adopted in October 2022 and was used in assessing compensation for our executives, including those decisions that were made in February 2023, particularly related to salary and annual equity awards that are described in this proxy statement.
2022-2023 Compensation Peer Group
| AbbVie | BioMarin Pharmaceutical | Merck | ||
| Alnylam Pharmaceuticals | Bristol-Myers Squibb | Pfizer | ||
| Amgen | Eli Lilly | Regeneron Pharmaceuticals | ||
| BeiGene Ltd. | Gilead Sciences | Seagen (fka Seattle Genetics) | ||
| Biogen | Incyte Corporation | Vertex Pharmaceuticals |
In August 2023, based on ongoing analysis prepared byfrom Pay Governance, in December 2020 the Compensation Committee approvedmade updates to the updated compensation peer group belowthat informed decisions made in February 2024 and that will inform decisions for the remainder of 2020 and for 2021; however, this peer group was not used for setting compensation for our NEOs for 2020. In making changes to the peer group, the committee continued to assess the factors listed above against the evolutionrest of the Company, including the launch of the Company’s first commercial product—the Moderna COVID-19 Vaccine—the increase in the Company’syear. Updates were primarily aimed at removing and replacing companies whose market capitalization as well asand future anticipated revenue growth.revenues were more aligned to Moderna on these factors.
2023-2024 Compensation Peer Group
Primary Elements of Executive Compensation ProgramThe primary elements of our executive compensation program are:
We do not have a specific policy regarding the percentage allocation between short-term and long-term, or fixed and variable, compensation elements. Our executive officers, including our NEOs, are also eligible to participate in our standard employee benefit plans, such as our health and welfare benefits plans, our employee stock purchase plan and our 401(k) Plan on the same basis as our other employees. In addition, as described below, our executive officers, including our NEOs, are entitled to certain change-in-control severance payments and benefits pursuant to our Executive Severance Plan, described herein. Base SalaryWe pay base salaries to our executive officers, including our NEOs, as the fixed portion of their compensation to provide them with a reasonable degree of personal income, and to attract and retain top-performing individuals. At the time of hire, base salaries are determined for our executive officers, including our NEOs, based on the factors described in “Governance of Executive Compensation Program—Compensation-Setting Factors” above. Typically, at the beginning of each year, the Compensation Committee reviews base salaries for our executive officers, including our NEOs, based on such factors to determine if an increase is appropriate. In addition, base salaries may be adjusted in the event of a promotion or significant change in
Salary increases for each of our NEOs were reviewed, approved and took effect in February
Short-Term Incentive CompensationWe provide short-term incentive compensation opportunities to our executive officers, including our NEOs, in the form of annual cash bonuses to drive our short-term success. Our annual cash bonuses are tied to the achievement of annual corporate and individual performance goals pursuant to the Company’s Senior Executive Cash Incentive Bonus Plan (the Corporate and Individual Performance GoalsAt the beginning of each year, the Compensation Committee discusses with the CEO the annual corporate performance objectives that are intended to be the most significant drivers of our short-term and long-term success. In addition, at the beginning of each year, our CEO, in consultation with each of the other executive officers, establishes individual performance goals for each of the other executive officers, including our other NEOs. The individual performance goals are generally designed to align the goals of our executive officers, including our NEOs, and his or her department with the corporate goals. The CEO discusses with the Compensation Committee his overall goals for the year which are in line with the overall corporate objectives but also include individual goals and action plans. The CEO’s goals and performance are ultimately evaluated, and his bonus is approved, by the full Board, with input from the Compensation Committee. At the beginning of the year after the corporate performance objectives are established, the Compensation Committee, after reviewing management’s self-assessment, evaluates specific achievements that are designed to advance the prior year’s corporate objectives, and our overall success in the prior year, and determines
CEO evaluates the other executive officers’, including the other NEOs’, achievement of their prior year’s individual performance goals, and makes recommendations for total percentage achievement level. The Compensation Committee considers our CEO’s recommendations, and independently reviews and approves the total percentage achievement level for each of the other executive officers, including our other NEOs. Target Annual BonusesAt the time of hire, the target annual bonus is determined for each of our executive officers, including our NEOs, and at the beginning of each year, the Compensation Committee reviews and approves the
target annual bonus for each such individual. The Compensation Committee considers the factors described in “Governance of Executive Compensation Program—Compensation-Setting Factors” above, with an emphasis on market data from our compensation peer group for comparable positions while also factoring internal parity. Target annual bonuses represent a specific percentage of annual base salary. 2023 Corporate Objectives
For each of these corporate objectives, our Compensation Committee In February
Set forth below is a
● = At Target ▲= Above Target ▼= Below Target ◆ = At Maximum ✖= Below Threshold ** Not disclosed; competitively sensitive
● = At Target ▲= Above Target ▼= Below Target ◆ = At Maximum ✖= Below Threshold ** Not disclosed; competitively sensitive
2023 Annual Bonus DeterminationAt the beginning of each year, the Compensation Committee reviews the target annual bonuses of our executive officers, including our NEOs. The Compensation Committee considered the factors described in “Governance of Executive Compensation Program—Compensation-Setting Factors” above, particularly market data from the companies in our compensation peer group. For In February
The individual performance factor for each NEO
Long-Term Incentive Compensation
2023 Equity AwardsThe value of At its February 2023 meeting, the Compensation Committee
The target equity values below
The equity awards granted to our NEOs in
Determination of 2021 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Performance Metric Goals |
| Performance Metric | Metric Weighting | Threshold (50% vesting) | Target (100% vesting) | Maximum (200% vesting) | Actual | Vesting Percentage | |
| Obtaining product approval under BLA from US FDA, or equivalent approval for commercial distribution in another major market | 25% | 1 (either in the US or the European Union) | 2 (approval in both the US and the European Union) | 4 or more (all major market countries) | ▲
| COVID-19 approval in:
1. US 2. Canada 3. EU 4. UK 5. Japan | 50% |
| Initiation of Phase 3 or registrational study for any non-COVID program | 50% | 1 | 3 | 4+ | ▲ | 1. INT Melanoma 2. Flu 3. RSV 4. Flu/COVID 5. CMV | 100% |
| Completing a non-vaccine Phase 1 or 2 trial producing positive readouts, other than in infectious disease vaccines | 25% | 1 | 2 | 3+ | ▲ | 1. INT Phase 2b (melanoma) 2. PA Phase 1/2 3. MMA Phase 1/2 4. GSD1a Phase 1 | 50% |
| Total Vesting | 200% | ||||||
| ▼ Threshold ● Target ▲ Maximum | |||||||
Based on the Committee’s approval of the 200% vesting, the following PSUs vested on February 14, 2024 for our products overNEOs that time horizon. In this first year,participated in the PSUs represent 25%program:
| Name | Number of PSUs Granted at Target | Grant Date Value(1) | Vesting Percentage | Earned Number of PSUs | Value at Vest(2) | ||
| Stéphane Bancel | 28,368 | $ | 3,749,966 | 200% | 56,736 | $ | 4,843,552 |
| Stephen Hoge, M.D. | 11,347 | 1,499,960 | 200% | 22,694 | 1,937,387 |
| (1) | Values represent grant date fair values calculated in accordance with FASB ASC Topic 718. |
| (2) | The value realized upon the vesting is calculated based on the closing market price of a share of our common stock on the date prior to the date of vesting. |
 | 2024 Proxy statement | 53 |
The table below summarizes the PSU awards that were granted in 2022, 2023 and 2024 that remain outstanding as of the overall grant date fair valueof this Proxy Statement, including an overview of the equity package awarded topre-established performance metrics for each award.
| Grant Year | 2022 | 2023 | 2024 | 2025 | 2026 | 2027 | |||
| 2022 | Key metrics: • Advancing our combination respiratory vaccine strategy • Diversifying our pipeline beyond respiratory vaccines • Establishing manufacturing plants • Building out our digital capabilities | ||||||||
| 2023 | Key metrics: • Obtaining approvals for respiratory disease programs and growing our U.S. market share • Advancing our individualized neoantigen therapy (INT) | ||||||||
| 2024 | Key Metrics: • Delivering on key externally communicated financial goals through 2026 • Executing on our late-stage pipeline beyond respiratory | ||||||||
The inclusion of long-term financial metrics into our Executive Committee members. For our CEO, the remaining 75% of his 2021 equity award was granted in stock options, andPSUs for the restfirst time in 2024 reflects our ongoing evolution and increased visibility into financial performance over a multi-year period as a commercial company, particularly as we execute on the anticipated launch of additional respiratory products. We also remain focused on expanding and diversifying our portfolio into non-respiratory therapeutic areas to drive long-term shareholder value.
2024 Long-Term Incentive Mix
At the February 2024 meeting, the Compensation Committee approved changes to the LTI mix for the CEO Compensation and other NEOs, shifting additional weighting towards PSUs to further tie our executive compensation program to our long-term financial and pipeline expansions objectives. We believe that this adjustment to the weighting of the Executive CommitteeLTI mix will continue to focus our executive team on achieving those strategic objectives that will lead to the annual award consistedcreation of 50% options and 25% RSUs. These options and RSUs have a four-year vesting scheduled, with 25% vesting on the first anniversarysustainable shareholder value.
Increased Weighting of the grant date, and the remainder vesting ratably over the next 12 quarters.PSUs for 2024 LTI Awards

 | 2024 Proxy statement | 54 |
Our executive officers, including our NEOs, are eligible to participate in the same employee benefit plans that are generally available to all of our employees, subject to the satisfaction of certain eligibility requirements, such asregular employees. These benefit plans include medical, dental, and life and disability insurance plans.plans, as well as well-being and time off programs. We pay, on behalf of our employees,sponsor a portion of the premiums for health, life and disability insurance. Certain employees at the vice president level and above, including the NEOs, are eligible for additional long-term disability insurance coverage.
Our executive officers, including our NEOs (other than Mr. Bancel, due to his ownership of more than 5% of our outstanding stock)Bancel), are eligible to participate in our 2018 Employee Stock Purchase Plan (the “ESPP”)ESPP) on the same basis as our other full-time U.S. employees. The ESPP is a broad-based stock ownership program that permits eligible employees to set aside a portion of their compensation during a six-month offering period and use such contributions to purchase shares of our common stock at a purchase price equal to 85% of the lower of the fair market value of the shares on the first business day of the offering period or the last business day of the purchase period. During 2020, none of our NEOs2023, Ms. Garay was the only NEO that participated in the ESPP.ESPP.
We maintain a tax-qualified retirement401(k) plan that provides eligible U.S. employees with an opportunity to save for retirement on a tax-advantaged basis.is tax-qualified under Section 401(a) of the Internal Revenue Code of 1986, as amended (the Code), and is tax-exempt under Section 501(a) of the Code. Plan participants can defer eligible compensation subject to applicable annual Internal Revenue Code of 1986, as amended (the “Code”) limits. We provide a matching contribution of 50%up to 4.5% (100% of employee contributions up to 6%the first 3% of compensation, which is 100% vested when contributed. The 401(k) plan is intended to be qualified under Section 401(a)and then 50% of the Code with the 401(k) plan’s related trust intended to be tax exempt under Section 501(a)next 3% of the Code.compensation). Once contributions are made, they are fully vested. As a tax-qualified retirement plan, contributions to the 401(k) plan and earnings on those contributions are not taxable to the employees until distributed from the 401(k) plan. Contributions can also be made on a Roth basis.
We provided limited perquisites to our named executive officersNEOs in 2020,2023, consisting primarily of security services and increased long-term disability insurance coverage, as well as reimbursing and paying related taxes for relocation benefits, reimbursement of housing and commuting expenses and other de minimis benefits.expenses. We believe that providing such perquisites was appropriate to assist our NEOs in the performance of their duties, to make our NEOs more efficient and effective and for recruitment and retention purposes.
In response to the increased profile of our Company and our executives as we pursued the development of a vaccine against COVID-19, beginning in 2020, the Company authorized the provision of personal and home security services to certain of our executives, including some of the NEOs. These services continued into 2023 for certain executives in response to the ongoing, heightened risk environment faced by these executives.
moderna 2021 Proxy statement |41
Employment Arrangements with our NEOs
Employment Offer Letters and Non-Compete, Non-Solicitation and Confidentiality Agreements
We generally enter into employment offer letters with new hires, including each of our NEOs when they joined the Company. These offer letters set forth the basic terms and conditions of their employment, including initial base salary, initial equity awards, eligibility to participate in our standard employee benefits plans, the at-will employment relationship and, in certain cases, a one-time signing bonus.bonus and relocation benefits. These offer letters also require that each NEO execute our standard employee confidentiality and assignment agreement. Each of our NEOs is subject to our standard non-competition, non-solicitation, confidentiality, and assignment agreement, which provides for a perpetual post-termination confidentiality covenant as well as post-termination non-competition and non-solicitation of customers, employees, and consultants covenants for one year following termination.
On May 5, 2020, Dr. Kim and the Company entered into an Executive Retention and Separation Agreement (the “Kim Retention and Separation Agreement”), setting forthRelease
In December 2023, we announced that following a restructuring of the terms of Dr. Kim’s continued services asCompany’s commercial organization and reporting lines, Arpa Garay, the Company’s Chief FinancialCommercial Officer, would be departing the Company, and that effective as of December 8, 2023, Ms. Garay would no longer serve in that role or as an executive officer of the Company. Ms. Garay has been asked to remain on as an employee of the Company through June 2024, and to serve in an advisory capacity to Mr. Bancel and Dr. Kim’s separation from employment on, August 23, 2020. UnderHoge to assist with the Kim Retentiontransition. At the time of Ms. Garay’s departure, she will be entitled to benefits under the Company’s Amended and Separation Agreement, Dr. Kim’s annual base salary continued to be no less than $582,000, except for across the board salary reductions impacting all executives proportionately. Under the agreement, Dr. Kim was also no longer eligible to participate in the Company’sRestated Executive Severance Plan, (as defined belowas amended on February 23, 2023 (Executive Severance Plan), subject to her execution of a separation agreement and release of claims in form and manner satisfactory to the Company, as disclosed under “Executive Severance Plan”) and instead became below. The benefits to which Ms. Garay will be entitled tounder the benefits and payments set forth in the Kim Retention and Separation Agreement. The Company agreed, subject to certain conditions, to pay Dr. Kim a cash lump sum in an amount equal to sixExecutive Severance Plan upon departure include: (i) twelve months of Dr. Kim’s annual base salary ($291,000)850,000), (ii) payment of her bonus for the year of departure at target ($765,000); and a one-time cash lump sum(iii) COBRA coverage for 12 months. Ms. Garay will also be provided accelerated vesting of 15% of any outstanding and unvested time-based equity awards, in an amount equal to 50% of Dr. Kim’s annual target bonus (i.e., $145,500). Under the Kim Retention and Separation Agreement, his options to purchaseaccordance with the Company’s common stock or other equity grantedguidelines governing involuntary terminations and up to Dr. Kim under the Company’s equity plans as of the12 months from her final date of the agreement continuedemployment to be governed by the terms and conditions of such plans and the applicable award agreements, and they were not otherwise accelerated.
Tal Zaks – Executive Retention Agreement
On March 29, 2020, Dr. Zaks and the Company entered into an Executive Retention Agreement (the “Zaks Retention Agreement”), setting forth the terms of Dr. Zaks’ continued services as the Company’s Chief Medical Officer through at least September 30, 2021 (the “Retention Date”). The Zaks Retention Agreement will be effective through the Retention Date or the last date of Dr. Zaks’ employment, if different, as set forth therein (the “Retention Period”).
During the Retention Period, Dr. Zaks’ base salaryexercise any vested but unexercised stock options. Ms. Garay will continue to be as set by the Company’s CEOeligible for her salary, benefits and the Compensation Committee and subject to periodic review and adjustments at the discretionvesting of the committee. During the Retention Period, Dr. Zaks will also remain eligible to participate in the Company’s Executive Severance Plan (as defined below under “Executive Severance Plan”) subject to the terms and conditions thereof. Provided that Dr. Zakstime-based
 | 2024 Proxy statement | 55 |
equity awards while she remains continuously employed by the Company, throughbut she will not be eligible for an equity award in 2024 or for a bonus for 2024 beyond the Retention Date, or in the event that Dr. Zaks’ employmentpayments to which she is terminated by the Company without Cause (as defined inotherwise entitled under the Executive Severance Plan) prior to the Retention Date, the Company will pay Dr. Zaks a one-time cash bonus of $1,000,000 (the “Retention Bonus”), subject to tax withholding under applicable law. Upon Dr. Zaks’ termination of employment on or after the Retention Date for any reason other than for Cause or in the event that the Company terminates Dr. Zaks’ employment without Cause prior to the Retention Date, then subject to Dr. Zaks’ agreement to a general release and certain other standard terms and conditions, any options to purchase the Company’s common stock granted to Dr. Zaks under the Company’s equity plans, to the extent vested, exercisable and outstanding immediately prior to such termination, will remain exercisable for two years following the date of such termination (but in no event later than the original expiration date applicable to such option).
On February 23, 2021, Dr. Zaks and the Company entered into an Amended and Restated Executive Retention Agreement (the “Amended Zaks Retention Agreement”), which modified the original Zaks Retention Agreement. Under the Amended Zaks Retention Agreement, if a successor Chief Medical Officer is appointed prior to September 30, 2021, Dr. Zaks will continue to serve as a Special Advisor to our CEO through that date, and as additional consideration a pro rata share of Dr. Zaks’ 2020 RSU grant, equivalent to 11,449 shares, would vest as of the Retention Date.Plan.
moderna 2021 Proxy statement |42
We believe that the severance payments and benefits provided under the Executive Severance Plans are appropriate in light of the post-employment compensation protections available to similarly-situatedsimilarly situated executive officers at companies in our compensation peer group and are an important component of each executive officer’s overall compensation as they help us to attract and retain our key executives who could have other job alternatives that may appear to them to be more attractive absent these protections.
In addition, we believe it is appropriate to provide enhanced severance benefits in connection with certain employment terminations occurring in connection with a change in control in order to encourage our executive officers to remain employed with us during an important time when their prospects for continued employment following the transaction are often uncertain. The primary purpose of these arrangements is to keep our most senior executive officers focused on pursuing potential corporate transactions that are in the best interests of our stockholdersshareholders regardless of whether those transactions may result in their own job loss. All of our NEOs other than Dr. Kim, are eligible for the Executive Severance Plan. Separate from the Executive Severance Plan, our NEOs are also covered by the Company’s guidelines regarding involuntary terminations, which provide for accelerated vesting of 15% of any outstanding and unvested time-based awards if termination occurs following the first anniversary of the new hire grant date.
The Executive Severance Plan provides that upon a termination of employment by us other than for “cause,“Cause,” death, or “disability,“Disability,” or upon a resignation by an eligible participant for “good reason”“Good Reason” (in each case, as defined in the Executive Severance Plan), in either case outside of the “change in control period” (i.e., the period beginning on the date of a “change in control” (as defined in the Executive Severance Plan) and ending on the one-year anniversary of the change in control), the participant will be entitled to receive, subject to the execution and delivery of a separation agreement and release containing, among other provisions, an effective release of claims in favor of the Company and reaffirmation of the “restrictive covenants agreement” (as defined in the Executive Severance Plan):
a severance amount equal to 12 months of the participant’s annual base salary in effect immediately prior to such termination, payable over 12 months,
| (i) | a severance amount equal to 12 months of the participant’s annual base salary in effect immediately prior to such termination, payable over 12 months in the form of salary continuation, |
| (ii) | an amount equal to the participant’s annual target bonus in effect immediately prior to such termination, payable over 12 months, and |
| (iii) | up to 12 monthly cash payments equal to the monthly employer contribution that we would have made to provide health insurance for the applicable participant if he or she had remained employed by us, based on the premiums as of the date of termination. |
an amount equal to (A) the participant’s annual target bonus in effect immediately prior to such termination, multiplied by (B) a fraction with a numerator equal to the number of full weeks elapsed in the then-current fiscal year prior to the date of termination and with a denominator equal to 52, payable over 12 months, and
up to 12 monthly cash payments equal to the monthly employer contribution that we would have made to provide health insurance for the applicable participant if he or she had remained employed by us, based on the premiums as of the date of termination.
Termination in connection with a change in control
The Executive Severance Plan also provides that upon a termination of employment by us other than for cause,Cause, death, or disabilityDisability or upon a resignation by an eligible participant for good reason,“Good Reason,” in either case within the change in control period, the participant will be entitled to receive, in lieu of the payments and benefits described above and subject to the execution and delivery of a separation agreement and release containing, among other provisions, an effective release of claims in favor of the Company and reaffirmation of the restrictive covenants agreement:
| (i) | a lump sum payment equal to 150% of the participant’s annual base salary in effect immediately prior to such termination (or the participant’s annual base salary in effect immediately prior to the change in control, if higher) paid in a cash lump sum as severance pay, |
| (ii) | a lump sum payment equal to 150% of the participant’s annual target bonus for the year such termination takes place (or the participant’s annual target bonus in effect immediately prior to the change in control, if higher) (the Applicable Bonus), |
| (iii) | a lump sum payment equal to (A) the participant’s Applicable Bonus multiplied by (B) a fraction with a numerator equal to the number of full weeks elapsed in the then-current fiscal year prior to the date of termination and with a denominator equal to 52, |
| (iv) | a lump sum amount equal to the monthly employer contribution that we would have made to provide health insurance for the participant if he or she had remained employed by us for 18 months following the date of termination, based on the premiums as of the date of termination, |
| (v) | for all outstanding and unvested equity awards of the Company that are subject to time-based vesting held by the named executive officer, full accelerated vesting of such awards, and |
| (vi) | for any performance-based equity awards, pro-rated acceleration based on the better of target and actual performance against the performance metrics. |
 | 2024 Proxy statement | 56 |
a lump sum cash severance amount equal to 150% of the participant’s annual base salary in effect immediately prior to such termination (or the participant’s annual base salary in effect immediately prior to the change in control, if higher),
a lump sum amount equal to 150% of the participant’s annual target bonus in effect immediately prior to such termination (or the participant’s annual target bonus in effect immediately prior to the change in control, if higher) (the “Applicable Bonus”),
a lump sum amount equal to (A) the participant’s Applicable Bonus multiplied by (B) a fraction with a numerator equal to the number of full weeks elapsed in the then-current fiscal year prior to the date of termination and with a denominator equal to 52,
a lump sum amount equal to the monthly employer contribution that we would have made to provide health insurance for the participant if he or she had remained employed by us for 18 months following the date of termination, based on the premiums as of the date of termination, and
for all outstanding and unvested equity awards of the Company that are subject to time-based vesting held by the named executive officer, full accelerated vesting of such awards.
| Back to Contents |
The payments and benefits provided under the Executive Severance Plan in connection with a change in control may not be eligible for a federal income tax deduction by us pursuant to Section 280G of the Code. These payments and benefits may also subject an eligible participant, including the named executive officers, to an excise tax under Section 4999 of the Code. If the payments or benefits payable to an eligible participant in connection with a change in control would be subject to the excise tax imposed under Section 4999 of the Code, then those payments or benefits will be reduced if such reduction would result in a greater net after-tax benefit to the applicable participant.
moderna 2021 Proxy statement |43
Equity-Related Policies and Practices
We have adopted an Equity Award Grant Policy that sets forth the process and timing for us to follow when we grant equity awards for shares of our common stock to our employees, including our executive officers, or advisors or consultants pursuant to any of our equity compensation plans. Pursuant to the policy, all grants of equity awards must be approved in advance by our Board of Directors, the Compensation Committee or, subject to the delegation requirements in the policy, our CEO.CEO or CHRO. The equity award granting authority delegated to our CEO and CHRO applies to employees at the senior vice president level and below and to equity awards within the specific ranges set forth in the policy. All equity awards for our Executive Committee members must be approved by the Compensation Committee or the full Board.
Generally, equity awards are granted on the following regularly scheduled basis as set forth in the policy:
Equity awards granted by our CEO in connection with the hiring of a new employee are effective generally on the first Monday that is trading day in a month following the hiring of the employee (or the same date, if starting on the first Monday of the month).
| • | Equity awards granted in connection with the hiring of a new employee are generally awarded on the fifth day of the month immediately following the month during which each new employee is hired. |
| • | Equity awards granted by our Board or the Compensation Committee in connection with the promotion of an existing employee or the engagement of a new consultant are effective on the date of approval by our Board or the Compensation Committee, as applicable, or such later date as specified in such approval. Our Board and the Compensation Committee retain the discretion to grant equity awards at other times to the extent appropriate in light of the circumstances of such awards. |
| • | Equity awards granted to existing employees (other than in connection with a promotion) will generally be granted, if at all, on an annual basis, including an annual award to all employees that is generally granted at the end of the second trading day following the filing of our Annual Report on 10-K with the SEC; additional grants for high-performing employees may be granted to certain employees at other times during the year. |
Equity awards granted by our Board of Directors or the Compensation Committee in connection with the hiring of a new employee or the promotion of an existing employee or the engagement of a new consultant are effective on the date of approval by our Board of Directors or the Compensation Committee, as applicable, or such later date as specified in such approval. Our Board of Directors and the Compensation Committee retain the discretion to grant equity awards at other times to the extent appropriate in light of the circumstances of such awards.
Equity awards granted to existing employees (other than in connection with a promotion) will generally be granted, if at all, on a periodic basis, including an annual award to all employees and a late year grant to certain employees.
In addition, the policyEquity Award Grant Policy sets forth the way our equity awards will be priced. If the grant of RSUs is denominated in dollars, the number of shares subject to each RSU award will be determined by dividing the value of such award by the average closing market price on the Nasdaq Global Select Market of a share of our common stock onover the preceding 20 trading days, up to and including the last trading day immediately preceding the effective date of grant (the 20-trading day trailing average price), and if the grant of an option is denominated in dollars, the number of shares subject to such option will be determined by dividing the value of such valueaward by the product of (i) the 20-trading day trailing average price and (ii) the Black-Scholes ratio, which is calculated using the Black-Scholes value of an option for oneon the grant date divided by the closing market price on the Nasdaq Global Select Market of a share of our common stock on the effective date of grant. The per share exercise price of all stock options will be at least equal to the closing market price on the Nasdaq Global Select Market of a share of our common stock on the effective date of grant.
In 2019, the Compensation Committee adopted a Stock Ownership Policy, which was subsequently amended in February 2021. As amended, the Stock Ownership Policy requires that by the fifth anniversary of the original effectiveness date of the policy (i.e., December 31, 2024), or the fifth anniversary of an individual becoming subject to the policy (whichever is later), that individual is required to hold a number of shares of Moderna stock equivalent in value to a multiple of the individual’s salary or, in the case of directors, their annual cash retainer, as follows:
CEO: 7 times annual salary
| • | CEO: 7 times annual salary |
| • | President: 6 times annual salary |
| • | Other Executive Committee members: 3 times annual salary |
| • | Directors: 6 times annual cash retainer |
President: 6 times annual salary
Other Executive Committee members: 3 times annual salary
Directors: 6 times annual cash retainer
In February 2021, the Stock Ownership Policy was revised by the Compensation Committee to provide that only owned shares would count toward satisfaction of the ownership requirement, eliminating credit previously granted for the value of vested but unexercised stock options. Until the requirements are met, covered individuals who were subject to the policy as of December 31, 2020 are required to hold 100% of any stock underlying vested RSU awards until the requirements are met, and individuals who are first subject to the policy on or after January 1, 2021 are required to hold 50% of any stock underlying vested RSU awards until the requirements are met.
 | 2024 Proxy statement | 57 |
As of December 31, 2020,2023 each of Mr. Bancel and Dr. Hoge owned more than the required value of sharesamount of Moderna common stock.
In February 2021, our Board of Directors adopted a clawback policy applicable to all performance-based compensation granted to members of our Executive Committee, beginning in 2021. The policy grants the Board or Compensation Committee discretion to recoup any performance-based compensation paid in excess of what otherwise should have been delivered due to the Executive Committee member’s misconduct that resulted in a financial restatement. In addition, the policy grants the Board or Compensation Committee discretion to recoup performance-based compensation in the event that an Executive Committee member’s detrimental conduct causes material financial, operational or reputational harm to the Company. In May 2023, our clawback policy was revised to reflect updated listing standard rules from Nasdaq and to require that the Company seek a clawback of any excess performance-based compensation that was paid to our Executive Committee due to an error or misstatement in our financial statements, regardless of fault or misconduct, other than in limited circumstances.
moderna 2021 Proxy statement |44
Policy Prohibiting Hedging and Pledging
Our Insider Trading Policy prohibits our executive officers, the non-employee members of our Board of Directors and certain designated employees who in the course of the performance of their duties have access to material, nonpublic information regarding the Company from engaging in the following transactions:
selling any of our securities that they do not own at the time of the sale (a “short sale”);
| • | selling any of our securities that they do not own at the time of the sale (a “short sale”); |
| • | buying or selling puts, calls, other derivative securities of the Company or any derivative securities that provide the economic equivalent of ownership of any of our securities or an opportunity, direct or indirect, to profit from any change in the value of our securities or engaging in any other hedging transaction with respect to our securities at any time; |
| • | using our securities as collateral in a margin account; and |
| • | pledging our securities as collateral for a loan (or modifying an existing pledge). |
buying or selling puts, calls, other derivative securities of the Company or any derivative securities that provide the economic equivalent of ownership of any of our securities or an opportunity, direct or indirect, to profit from any change in the value of our securities or engaging in any other hedging transaction with respect to our securities at any time;
using our securities as collateral in a margin account; and
pledging our securities as collateral for a loan (or modifying an existing pledge).
As of the date of this Proxy Statement, none of our NEOs had previously sought or obtained approval from the Compensation Committee to engage in any hedging or pledging transaction involving our securities.
moderna 2021 Proxy statement |45
 | 2024 Proxy statement | 58 |
20202023 Summary Compensation Table
The following table provides information regarding the total compensation awarded to, earned by, and paid to our named executive officersNEOs for services rendered to us for the years set forth below. Please note that in certain years these individuals were not NEOs and as such we are not including their compensation for those years.
Name and Principal Position(1) | Year | Salary ($) | Bonus ($) | Stock Awards ($) | Option Awards ($) | All Other Compensation ($) | Total ($) | ||||||||||||
Stéphane Bancel Chief Executive Officer | 2020 | $ | 945,673 | $ | 1,900,000 | $ | — | $ | 9,000,000 | $ | 1,009,602 | $ | 12,855,275 | ||||||
2019 |
| 921,217 |
| 1,017,500 |
| — |
| 7,000,000 |
| 9,490 |
| 8,948,207 | |||||||
2018 |
| 863,077 |
| 1,800,000 |
| — |
| 55,935,768 |
| 9,639 |
| 58,608,484 | |||||||
David Meline Chief Financial Officer | 2020 |
| 334,616 |
| 339,344 |
| — |
| 8,600,000 |
| — |
| 9,273,960 | ||||||
|
|
|
|
|
|
|
|
|
|
|
|
| |||||||
Lorence Kim, M.D. Former Chief Financial Officer | 2020 |
| 418,211 |
| 145,000 |
| — |
| 2,500,000 |
| 330,775 |
| 3,393,986 | ||||||
2019 |
| 560,401 |
| 278,685 |
| — |
| 2,500,000 |
| 258,479 |
| 3,597,565 | |||||||
2018 |
| 542,769 |
| 450,450 |
| — |
| 7,816,512 |
| 228,386 |
| 9,038,117 | |||||||
Stephen Hoge, M.D. President | 2020 |
| 617,366 |
| 621,000 |
| 1,000,000 |
| 3,000,000 |
| 24,930 |
| 5,263,296 | ||||||
2019 |
| 595,724 |
| 396,000 |
| — |
| 4,000,000 |
| 9,490 |
| 5,001,214 | |||||||
2018 |
| 570,004 |
| 629,200 |
| — |
| 3,517,431 |
| 9,639 |
| 4,726,274 | |||||||
Tal Zaks Chief Medical Officer | 2020 |
| 548,712 |
| 552,000 |
| 750,000 |
| 2,250,000 |
| 152,269 |
| 4,252,981 | ||||||
2019 |
| 530,630 |
| 293,150 |
| — |
| 4,000,000 |
| 8,646 |
| 4,832,426 | |||||||
Juan Andres Chief Technical Operations and Quality Officer | 2020 |
| 548,712 |
| 662,400 |
| 750,000 |
| 2,250,000 |
| 36,711 |
| 4,247,823 | ||||||
2019 |
| 530,615 |
| 322,465 |
| — |
| 3,000,000 |
| 9,490 |
| 3,862,570 | |||||||
(1) Mr. Meline was hired by the Company on June 8, 2020 and was not an NEO for 2019 or 2018. Dr. Zaks and Mr. Andres were not NEOs in 2018. Dr. Kim was an employee through August 23, 2020. | |||||||||||||||||||
| Name and Principal Position(1) | Year | Salary | Non-Equity Plan Incentive Compensation | Bonus | Stock Awards(2) | Option Awards(2) | All Other Compensation | Total | |||||||||||||||
Stéphane Bancel | 2023 | $ | 1,563,462 | $ | 1,913,625 | $ | — | $ | 3,129,194 | $ | 9,387,713 | $ | 1,074,520 | $ | 17,068,514 | ||||||||
| 2022 | 1,423,077 | 2,700,000 | — | 3,597,152 | 10,791,857 | 851,562 | 19,363,648 | ||||||||||||||||
| 2021 | 990,385 | 1,500,000 | — | 3,750,000 | 11,250,000 | 665,354 | 18,155,739 | ||||||||||||||||
James Mock | 2023 | 792,308 | 583,200 | — | 1,460,282 | 1,460,295 | 21,100 | 4,317,185 | |||||||||||||||
| 2022 | 227,885 | 259,644 | 1,000,000 | 2,919,660 | 2,919,680 | 4,327 | 7,331,196 | ||||||||||||||||
Stephen Hoge, M.D. | 2023 | 1,042,308 | 850,500 | — | 2,711,792 | 2,711,966 | 22,600 | 7,339,166 | |||||||||||||||
| 2022 | 953,846 | 1,440,000 | — | 3,117,492 | 3,117,579 | 17,735 | 8,646,652 | ||||||||||||||||
| 2021 | 684,808 | 819,000 | — | 3,000,000 | 3,000,000 | 299,624 | 7,803,432 | ||||||||||||||||
Arpa Garay | 2023 | 842,308 | 619,650 | — | 1,460,282 | 1,460,295 | 63,413 | 4,445,948 | |||||||||||||||
| 2022 | 458,462 | 508,932 | 1,500,000 | 2,502,713 | 2,502,742 | 203,303 | 7,676,152 | ||||||||||||||||
Shannon Thyme Klinger Chief Legal Officer and Corporate Secretary | 2023 | 784,616 | 583,200 | — | 1,460,282 | 1,460,295 | 24,103 | 4,312,496 | |||||||||||||||
| 2022 | 692,590 | 756,000 | — | 1,112,728 | 1,112,705 | 47,173 | 3,721,196 | ||||||||||||||||
| 2021 | 381,096 | 344,000 | 250,000 | 6,000,000 | 4,000,000 | 514,051 | 11,489,147 | ||||||||||||||||
| (1) | Mr. Mock and Ms. Garay were hired on September 6, 2022 and May 31, 2022, respectively. Neither was an NEO for 2021; Ms. Klinger was not an NEO for 2022. |
| (2) | The amounts reported represent the aggregate grant date fair value of the RSUs, PSUs and stock options, respectively, awarded to our NEOs in the year ended December 31, 2023, calculated in accordance with FASB ASC Topic 718. Such grant date fair values do not take into account any estimated forfeitures. The assumptions used in calculating the grant date fair value of the equity awards reported in these columns are set forth in Note 12 to our Consolidated Financial Statements for the year ended December 31, 2023 included in our Annual Report. The amounts reported in these columns reflect the grant date fair value for each award, and, together, differ from the targeted amounts for these grants, due to the fact that a 20-trading day trailing average closing price convention is used for calculating the number of RSUs, PSUs and stock options granted. The grant date fair value of the PSUs are based on the probable outcome of the applicable performance metrics. The amounts reported in this column reflect the accounting cost for these awards and do not correspond to the actual economic value that may be received by the NEO upon the exercise of the stock options or any sale of the underlying shares of common stock. |
Amounts represent the actual amount of base salary paid for each NEO during the applicable year. NEOs and other employees are generally assessed for potential salary increases at the beginning of each year. Percentage salary increases for each of our NEOs (other than Mr. Meline, whose salary was fixed at $600,000 upon hiring) were approved and took effect in February 20202023 as follows: Mr. Bancel (2.7%- 5%, to $950,000), Kim (3.4%$1,575,000, Dr. Hoge - 5%, to $582,000), Hoge (3.5%,$1,050,000, Mr. Mock - 6.7% to $621,000), Zaks (3.6%,$800,000; Ms. Garay - 6.3% to $552,000),$850,000; and Andres (3.6%,Ms. Klinger - 14.3% to $552,000). For more information, see$800,000. Salary increases were reflective of merit and market adjustments to better align each of these executive’s salaries to market in the discussioncase of Mr. Mock, Ms. Garay and Ms. Klinger, as well as cost-of-living adjustments applied across our broader employee base for each NEO under “Executive Summary” above.of these executives.
The amounts reported represent annual bonuses earned by our NEOs for services performed during 2020, 2019 and 2018, asthe applicable year, based on the achievement of Company and individual performance objectives, other than the 2020 bonus for Dr. Kim, which was paid pursuant to the Kim Retention and Severance Agreement.objectives. Target bonuses for our NEOs are set as a percentage of annual salary, and for 20202023 were maintained at 150% of salary for our CEO, 100% of salary for our CEOPresident, and 50%90% of salary for our other NEOs. Mr. Meline’s bonus was prorated due to his being employed for only part of the year. Dr. Kim was ineligible for a performance bonus due to his mid-year departure. For more information, see “Short-Term Incentive Compensation” above.
 | 2024 Proxy statement | 59 |
For Mr. Mock and Ms. Garay, in 2022, and for Ms. Klinger in 2021, the amount under “Bonus” represents amounts received as a signing bonus in connection with each executive’s hiring. These signing bonuses are subject to a clawback if the executive voluntarily departs the Company or is dismissed for cause within two years of the payment of the signing bonus.
The amount reported represents the aggregate grant date fair value of RSUs and PSUs awarded to the NEOs, calculated in accordance with Financial Accounting Standards Board (“FASB”) Accounting Standards Codification (“ASC”)FASB ASC Topic 718. Such grant date fair value does not take into account any estimated forfeitures. The assumptions used in calculating the grant date fair value of the RSUs and PSUs reported in this column are set forth in Note 1412 to our Consolidated Financial Statements for the year ended December 31, 20202023 included in our Annual Report. The amount reported in this column reflects the accounting cost for these RSUsequity awards and does not correspond to the actual economic value that may be received by the applicable NEO upon the vesting/settlement of the RSUs and PSUs or any sale of the underlying shares of common stock. The grant date fair value of the 2023 PSUs, calculated in accordance with FASB ASC Topic 718, assuming maximum achievement of the applicable performance metrics, are as follows: Mr. Bancel - $6,258,388; Dr. Hoge - $2,711,792; Mr. Mock, Ms. Garay and Ms. Klinger - $1,460,282. Amounts for Mr. Mock and Ms. Garay in 2022, and for Ms. Klinger in 2021, represent the value of new hire equity awards, which were set based upon market data for executives of comparable caliber and experience, while also taking into account the value of equity awards that each of them would forfeit by leaving their former employers to join Moderna.
moderna 2021 Proxy statement |46
The amounts reported represent the aggregate grant date fair value of the stock options awarded to the NEOs during 2020, 2019 and 2018, asthe applicable year, calculated in accordance with FASB ASC Topic 718. Such grant date fair values do not take into account any estimated forfeitures. The assumptions used in calculating the grant date fair value of the stock options reported in this column are set forth in Note 1412 to our Consolidated Financial Statements for the year ended December 31, 20202023 included in our Annual Report. The amounts reported in this column reflect the accounting cost for these stock options and do not correspond to the actual economic value that may be received by the NEOs upon the exercise of the stock options or any sale of the underlying shares of common stock. The amount shownAmounts for Mr. Meline reflects hisMock and Ms. Garay in 2022, and for Ms. Klinger in 2021, represent the value of new hire equity awards. The 2020 awardawards, which were set based upon market data for Dr. Kim was forfeited upon his departure fromexecutives of comparable caliber and experience, while also taking into account the Company.value of equity awards that each of them would forfeit by leaving their former employers to join Moderna.
Amounts reported represent $8,550 for matching contributions made byThe amounts set forth below provide a detailed breakdown of the Company in 2020 under its 401(k) plan for Messrs. Bancel, Kim, Hoge, Zaks and Andres.
For Dr. Kim for 2020,2023 amounts reported also reflect $291,000 in severance (see “Lorence Kim – Executive Retention and Separation Agreement,” above) and $31,225above for housing and commuting expenses and related tax-gross up payments for such expenses.All Other Compensation. These amounts consist of the following:
Amounts also include the following incremental expense recognized by the Company for the provision of personal and home security services to the following individuals: Mr. Bancel ($1,001,052); Dr. Hoge ($16,380); Mr. Andres ($28,161) and Dr. Zaks ($143,719). The Company began to provide such services to address increased security concerns as we pursued the development of a COVID-19 vaccine during 2020.
| • | 401(k) Match: Represents matching contributions to the 401(k) account for the named executive officer. |
| • | Relocation/Relocation Tax Expenses: Represents benefits paid on behalf of Ms. Garay in 2023 in connection with her relocation to the Boston area to begin employment with Moderna in 2022, and related tax gross-up payments for such expenses. |
| • | Other Compensation: Represents incremental costs borne by the Company for the provision of security services to the NEOs or members of their household in response to a heightened threat environment, and the amount of premiums paid on behalf of the NEOs for supplemental long-term disability coverage, which we offer to executives at the vice president level and above. For Mr. Bancel, the amount paid for security services in 2023 was $1,053,767. |
| Name | 401(k) Match | Relocation | Relocation Tax Expenses | Other Compensation | Total | |||||||||||||||
| Stéphane Bancel | $ | 14,850 | $ | — | $ | — | $ | 1,059,670 | $ | 1,074,520 | ||||||||||
| James Mock | 14,850 | — | — | 6,250 | 21,100 | |||||||||||||||
| Stephen Hoge, M.D. | 14,850 | — | — | 7,750 | 22,600 | |||||||||||||||
| Arpa Garay | 14,850 | 21,769 | 21,466 | 5,328 | 63,413 | |||||||||||||||
| Shannon Thyme Klinger | 14,850 | — | — | 9,253 | 24,103 | |||||||||||||||
 | 2024 Proxy statement | 60 |
The following table – also known as the Grants of Plan-Based Awards Table – sets forth the individual awardsaward, including stock options, RSUs and PSUs, made to each of our NEOs during 2020.2023. For a description of the types of awards indicated below, please see our “Compensation Discussion and Analysis” above.
Name | Grant Date(1) | Number of Shares of Stock or Units (#)(2) | Number of Securities Underlying Options (#)(3) | Exercise or Base Price of Option Awards ($/share)(4) | Grant Date Fair Value of Stock and Option Awards ($)(5) | ||
Stéphane Bancel | February 28, 2020 | — | 644,480 | $ | 25.93 | $ | 9,000,000 |
David Meline | July 6, 2020 | — | 271,303 |
| 59.15 |
| 8,600,000 |
Lorence Kim, M.D. | February 28, 2020 | — | 179,022 |
| 25.93 |
| 2,500,000 |
Stephen Hoge, M.D. | February 28, 2020 | 38,565 | 214,826 |
| 25.93 |
| 4,000,000 |
Tal Zaks, M.D. | February 28, 2020 | 28,924 | 161,120 |
| 25.93 |
| 3,000,000 |
Juan Andres | February 28, 2020 | 28,924 | 161,120 |
| 25.93 |
| 3,000,000 |
(1) All grants were approved by the Compensation Committee on February 25, 2020, with a grant date of February 28, 2020, other than the new hire award for Mr. Meline, which was approved by the Compensation Committee on May 29, 2020, with a grant date of July 6, 2020, consistent with the Company’s grant date convention for new hires under the Company’s Equity Award Grant policy. (2) Each RSU is subject to time-based vesting, as described in the footnotes to the “Outstanding Equity Awards at 2020 Year-End Table” below. (3) Each stock option is subject to time-based vesting, as described in the footnotes to the “Outstanding Equity Awards at 2020 Year-End Table” below. (4) Based upon the closing sale price of our common stock as reported on the Nasdaq Global Select Market on the date of grant. (5) The amounts reported represent the aggregate grant date fair value of the stock options and RSUs, as applicable, awarded to the NEOs during 2020, calculated in accordance with FASB ASC Topic 718. Such grant date fair values do not take into account any estimated forfeitures. The assumptions used in calculating the grant date fair value of the stock options and RSUs, as applicable, reported in this column are set forth in Note 14 to our Consolidated Financial Statements for the year ended December 31, 2020 included in our Annual Report. The amounts reported in this column reflect the aggregate accounting cost for these stock options and RSUs, and do not correspond to the actual economic value that may be received by the NEOs upon the exercise of the stock options, the vesting/settlement of the RSUs or any sale of the underlying shares of common stock. | |||||||
| Name | Grant Date(1) | Award Type | Estimated Future Payouts Under Performance Share Units (#)(2) | Restricted Stock Units (#)(3) | Stock Options (#)(4) | Stock Option Exercise Price(5) | Grant Date Fair Value of Awards(6) | |||||||||||||||
| Threshold | Target | Maximum | ||||||||||||||||||||
| Stéphane Bancel | February 28, 2023 | Annual Equity | 128,830 | $138.81 | $9,387,713 | |||||||||||||||||
| February 28, 2023 | Annual Equity | 11,271 | 22,543 | 45,086 | 3,129,194 | |||||||||||||||||
| James Mock | February 28, 2023 | Annual Equity | 20,040 | 138.81 | 1,460,295 | |||||||||||||||||
| February 28, 2023 | Annual Equity | 5,260 | 730,141 | |||||||||||||||||||
| February 28, 2023 | Annual Equity | 2,630 | 5,260 | 10,520 | 730,141 | |||||||||||||||||
| Stephen Hoge, M.D. | February 28, 2023 | Annual Equity | 37,217 | 138.81 | 2,711,966 | |||||||||||||||||
| February 28, 2023 | Annual Equity | 9,768 | 1,355,896 | |||||||||||||||||||
| February 28, 2023 | Annual Equity | 4,884 | 9,768 | 19,536 | 1,355,896 | |||||||||||||||||
| Arpa Garay | February 28, 2023 | Annual Equity | 20,040 | 138.81 | 1,460,295 | |||||||||||||||||
| February 28, 2023 | Annual Equity | 5,260 | 730,141 | |||||||||||||||||||
| February 28, 2023 | Annual Equity | 2,630 | 5,260 | 10,520 | 730,141 | |||||||||||||||||
| Shannon Thyme Klinger | February 28, 2023 | Annual Equity | 20,040 | 138.81 | 1,460,295 | |||||||||||||||||
| February 28, 2023 | Annual Equity | 5,260 | 730,141 | |||||||||||||||||||
| February 28, 2023 | Annual Equity | 2,630 | 5,260 | 10,520 | 730,141 | |||||||||||||||||
| (1) | All annual equity grants were approved by the Compensation Committee on February 14, 2023, with a grant date of February 28, 2023, for annual stock option, RSU and PSU grants (the second trading day following the filing of our Annual Report 10-K with the SEC). |
| (2) | Each PSU is subject to vesting upon a determination by the Compensation Committee that the goals thereunder have been met. This determination is expected to be made within two-and-a-half months of the conclusion of the performance period, which ends on December 31, 2025. |
| (3) | Each RSU is subject to time-based vesting, as described in the footnotes to the “Outstanding Equity Awards at 2023 Year-End” table below. |
| (4) | Each stock option is subject to time-based vesting, as described in the footnotes to the “Outstanding Equity Awards at 2023 Year-End” table below. |
| (5) | Based upon the closing price of our common stock as reported on the Nasdaq Global Select Market on the date of grant. |
| (6) | The amounts reported represent the aggregate grant date fair value of the stock options, RSUs and PSUs, as applicable, awarded to the NEOs during 2023, calculated in accordance with FASB ASC Topic 718. Such grant date fair values do not take into account any estimated forfeitures. The assumptions used in calculating the grant date fair value of the stock options, RSUs and PSUs, as applicable, reported in this column are set forth in Note 12 to our Consolidated Financial Statements for the year ended December 31, 2023 included in our Annual Report. The amounts reported in this column reflect the aggregate accounting cost for these equity awards, and do not correspond to the actual economic value that may be received by the NEOs upon the exercise of the stock options, the vesting/settlement of the RSUs or PSUs or any sale of the underlying shares of common stock. The grant date fair value of PSUs is based on probable achievement of the performance metrics at target. |
moderna 2021 Proxy statement |47
 | 2024 Proxy statement | 61 |
The table below sets forth information regarding outstanding equity awards held by our NEOs as of December 31, 2020. Dr. Kim, our former CFO, did not have any equity awards outstanding as of that date.2023.
Name | Grant Date(1) | Award Type | First Vesting Date | Number Exercisable/ Outstanding |
| Number Unexercisable/ Unvested |
|
| Option Exercise Price | Option Expiration Date |
| Market Value(2) |
Stéphane Bancel | 8/19/2013 | Options | 8/10/2016 | 4,587,155 | (3) | — |
| $ | 0.99 | 8/19/2023 | $ | 474,678,799 |
2/23/2016 | Options | 2/23/2017 | 688,073 | (3) | — |
|
| 10.90 | 2/23/2026 |
| 64,382,991 | |
8/10/2016 | Options | 8/10/2016 | 558,394 | (3) | — |
|
| 19.15 | 8/10/2026 |
| 47,642,176 | |
8/10/2016 | Options | 8/10/2016 | 193,321 | (3) | — |
|
| 19.15 | 8/10/2026 |
| 16,494,148 | |
2/23/2017 | Options | 2/22/2018 | 602,057 | (4) | 40,144 | (4) |
| 12.21 | 2/23/2027 |
| 59,249,464 | |
2/28/2018 | Options | 2/28/2019 | 415,706 | (5) | 501,725 | (5) |
| 14.22 | 2/28/2028 |
| 82,798,148 | |
12/6/2018 | Options | 6/13/2020 | 860,090 | (6) | 3,727,065 | (6) |
| 23.00 | 12/6/2028 |
| 373,715,518 | |
3/8/2019 | Options | 3/8/2020 | 259,696 | (4) | 333,896 | (4) |
| 20.93 | 3/8/2029 |
| 49,588,676 | |
2/28/2020 | Options | 2/28/2021 | — |
| 644,480 | (4) |
| 25.93 | 2/28/2030 |
| 50,617,459 | |
|
|
|
|
|
|
|
|
|
|
| 1,219,167,379 | |
David Meline | 7/6/2020 | Options | 7/6/2021 | — |
| 271,303 | (4) |
| 59.15 | 7/6/2030 |
| 12,295,452 |
|
|
|
|
|
|
|
|
|
|
| 12,295,452 | |
Stephen Hoge | 8/10/2016 | Options | 8/10/2016 | 677,431 | (3) | — |
|
| 0.99 | 8/19/2023 |
| 70,100,560 |
8/10/2016 | Options | 2/23/2017 | 366,972 | (3) | — |
|
| 10.90 | 2/23/2026 |
| 34,337,570 | |
8/10/2016 | Options | 8/10/2016 | 223,357 | (3) | — |
|
| 19.15 | 8/10/2026 |
| 19,056,819 | |
8/10/2016 | Options | 8/10/2016 | 96,660 | (3) | — |
|
| 19.15 | 8/10/2026 |
| 8,247,031 | |
2/23/2017 | Options | 2/22/2018 | 430,037 | (4) | 28,678 | (4) |
| 12.21 | 2/23/2027 |
| 42,321,046 | |
10/3/2017 | Options | 10/3/2018 | 1,032,108 | (7) | 802,754 | (7) |
| 12.21 | 10/3/2027 |
| 169,284,368 | |
2/28/2018 | Options | 2/27/2019 | 283,825 | (4) | 129,019 | (4) |
| 14.22 | 2/28/2028 |
| 37,259,171 | |
3/8/2019 | Options | 3/8/2020 | 148,397 | (4) | 190,798 | (4) |
| 20.93 | 3/8/2029 |
| 28,336,350 | |
2/28/2020 | Options | 2/28/2021 | — |
| 214,826 | (4) |
| 25.93 | 2/28/2030 |
| 16,872,434 | |
2/28/2020 | RSUs | 2/28/2022 | — |
| 38,565 | (8) |
|
|
|
| 4,028,886 | |
|
|
|
|
|
|
|
|
|
|
| 429,844,235 | |
Tal Zaks | 2/23/2017 | Options | 2/22/2017 | — |
| 11,478 | (4) |
| 12.21 | 2/23/2027 |
| 1,058,960 |
10/3/2017 | Options | 10/3/2018 | — |
| 194,952 | (4) |
| 12.21 | 10/3/2027 |
| 17,986,272 | |
2/28/2018 | Options | 2/27/2019 | — |
| 50,175 | (4) |
| 14.22 | 2/28/2028 |
| 4,528,294 | |
3/8/2019 | Options | 3/8/2020 | — |
| 190,798 | (4) |
| 20.93 | 3/8/2029 |
| 15,939,265 | |
2/28/2020 | Options | 2/28/2021 |
|
| 161,120 | (4) |
| 25.93 | 2/28/2030 |
| 12,654,365 | |
2/28/2020 | RSUs | 2/28/2022 |
|
| 28,924 | (8)(9) |
|
|
|
| 3,021,690 | |
|
|
|
|
|
|
|
|
|
|
| 55,188,846 | |
Juan Andres | 8/29/2017 | Options | 8/29/2018 | 512,799 | (4) | 172,023 | (4) |
| 12.21 | 8/29/2027 |
| 63,181,678 |
2/28/2018 | Options | 2/28/2019 | 47,301 | (4) | 21,506 | (4) |
| 14.22 | 2/28/2028 |
| 6,209,832 | |
3/8/2019 | Options | 3/8/2020 | 111,298 | (4) | 143,098 | (4) |
| 20.93 | 3/8/2029 |
| 21,252,242 | |
2/28/2020 | Options | 2/28/2021 | — |
| 161,120 | (4) |
| 25.93 | 2/28/2030 |
| 12,654,365 | |
2/28/2020 | RSUs | 2/28/2022 |
|
| 28,924 | (8) |
|
|
|
| 3,021,690 | |
|
|
|
|
|
|
|
|
|
|
| 106,319,806 |
| Name | Grant Date(1) | Award Type | First Vesting Date | Number Exercisable/ Vested | Number Unexercisable/ Unvested | Option Exercise Price | Option Expiration Date | Market Value(2) | |||||||||||
| Stéphane Bancel | 2/23/2016 | Options | 2/23/2017 | 688,073(3) | — | $ | 10.90 | 2/23/2026 | $ | 60,928,864 | |||||||||
| 8/10/2016 | Options | 8/10/2016 | 558,394(3) | — | 19.15 | 8/10/2026 | 44,839,038 | ||||||||||||
| 8/10/2016 | Options | 8/10/2016 | 193,321(3) | — | 19.15 | 8/10/2026 | 15,523,676 | ||||||||||||
| 2/23/2017 | Options | 2/22/2018 | 642,201(3) | — | 12.21 | 2/23/2027 | 56,025,615 | ||||||||||||
| 2/28/2018 | Options | 2/28/2019 | 903,096(4) | 14,335(4) | 14.22 | 2/28/2028 | 78,192,644 | ||||||||||||
| 12/6/2018 | Options | 6/13/2020 | 4,587,155(3) | — | 23.00 | 12/6/2028 | 350,688,000 | ||||||||||||
| 3/8/2019 | Options | 3/8/2020 | 593,592(3) | — | 20.93 | 3/8/2029 | 46,608,844 | ||||||||||||
| 2/28/2020 | Options | 2/28/2021 | 604,200(5) | 40,280(5) | 25.93 | 2/28/2030 | 47,382,170 | ||||||||||||
| 2/9/2021 | Options | 2/9/2022 | 99,071(5) | 45,034(5) | 179.52 | 2/9/2031 | — | ||||||||||||
| 3/5/2021 | PSUs | — | 28,368(7) | — | 2,821,198 | ||||||||||||||
| 3/1/2022 | Options | 3/1/2023 | 64,034(5) | 82,330(5) | 149.52 | 3/1/2032 | — | ||||||||||||
| 3/1/2022 | PSUs | — | 24,058(7) | — | 2,392,568 | ||||||||||||||
| 2/28/2023 | Options | 2/28/2024 | — | 128,830(5) | 138.81 | 2/28/2033 | — | ||||||||||||
| 2/28/2023 | PSUs | — | 22,543(7) | — | 2,241,901 | ||||||||||||||
| 707,644,518 | |||||||||||||||||||
| James Mock | 10/5/2022 | Options | 10/5/2023 | 10,969(5) | 32,907(5) | 125.62 | 10/5/2032 | — | |||||||||||
| 10/5/2022 | RSUs | 10/5/2023 | — | 17,432(5) | — | 1,733,612 | |||||||||||||
| 2/28/2023 | Options | 2/28/2024 | — | 20,040(5) | 138.81 | 2/28/2033 | — | ||||||||||||
| 2/28/2023 | RSUs | 2/28/2024 | — | 5,260(5) | — | 523,107 | |||||||||||||
| 2/28/2023 | PSUs | — | 5,260(7) | — | 523,107 | ||||||||||||||
| 2,779,826 | |||||||||||||||||||
| Stephen Hoge, M.D. | 8/10/2016 | Options | 8/10/2016 | 223,357(3) | — | 19.15 | 8/10/2026 | 17,935,567 | |||||||||||
| 8/10/2016 | Options | 8/10/2016 | 96,660(3) | — | 19.15 | 8/10/2026 | 7,761,798 | ||||||||||||
| 2/23/2017 | Options | 2/22/2018 | 458,715(3) | — | 12.21 | 2/23/2027 | 40,018,297 | ||||||||||||
| 10/3/2017 | Options | 10/3/2018 | 1,834,862(3) | — | 12.21 | 10/3/2027 | 160,073,361 | ||||||||||||
| 2/28/2018 | Options | 2/27/2019 | 412,844(3) | — | 14.22 | 2/28/2028 | 35,186,694 | ||||||||||||
| 3/8/2019 | Options | 3/8/2020 | 339,195(3) | — | 20.93 | 3/8/2029 | 26,633,591 | ||||||||||||
| 2/28/2020 | Options | 2/28/2021 | 201,398(5) | 13,428(5) | 25.93 | 2/28/2030 | 15,794,008 | ||||||||||||
| 2/28/2020 | RSUs | 2/28/2022 | — | 2,411(6) | — | 239,774 | |||||||||||||
| 2/9/2021 | Options | 2/9/2022 | 26,419(5) | 12,009(5) | 179.52 | 2/9/2031 | — | ||||||||||||
| 2/9/2021 | RSUs | 2/9/2022 | — | 2,612(5) | — | 259,763 | |||||||||||||
| 3/5/2021 | PSUs | — | 11,347(7) | — | 1,128,459 | ||||||||||||||
| 3/1/2022 | Options | 3/1/2023 | 18,497(5) | 23,785(5) | 149.52 | 3/1/2032 | — | ||||||||||||
| 3/1/2022 | RSUs | 3/1/2023 | — | 5,865(5) | — | 583,274 | |||||||||||||
| 3/1/2022 | PSUs | — | 10,425(7) | — | 1,036,766 | ||||||||||||||
| 2/28/2023 | Options | 2/28/2024 | — | 37,217(5) | 138.81 | 2/28/2033 | — | ||||||||||||
| 2/28/2023 | RSUs | 2/28/2024 | — | 9,768(5) | — | 971,428 | |||||||||||||
| 2/28/2023 | PSUs | — | 9,768(7) | — | 971,428 | ||||||||||||||
| 308,594,208 | |||||||||||||||||||
moderna 2021 Proxy statement |48
 | 2024 Proxy statement | 62 |
| Name | Grant Date(1) | Award Type | First Vesting Date | Number Exercisable/ Vested | Number Unexercisable/ Unvested | Option Exercise Price | Option Expiration Date | Market Value(2) | |||||||||||
| Arpa Garay | 6/5/2022 | Options | 6/5/2023 | 13,036(5) | 21,730(5) | 137.15 | 6/5/2032 | — | |||||||||||
| 6/5/2022 | RSUs | 6/5/2023 | — | 11,405(5) | — | 1,134,227 | |||||||||||||
| 2/28/2023 | Options | 2/28/2024 | — | 20,040(5) | 138.81 | 2/28/2033 | — | ||||||||||||
| 2/28/2023 | RSUs | 2/28/2024 | — | 5,260(5) | — | 523,107 | |||||||||||||
| 2/28/2023 | PSUs | — | 5,260(7) | — | 523,107 | ||||||||||||||
| 2,180,441 | |||||||||||||||||||
| Shannon Thyme Klinger | 6/7/2021 | Options | 6/7/2022 | 26,355(5) | 15,814(5) | 219.57 | 6/7/2031 | — | |||||||||||
| 6/7/2021 | RSUs | 6/7/2022 | — | 6,832(5) | — | 679,442 | |||||||||||||
| 6/7/2021 | RSUs | 6/7/2024 | — | 9,108(8) | — | 905,791 | |||||||||||||
| 3/1/2022 | Options | 3/1/2023 | 6,601(5) | 8,490(5) | 149.52 | 3/1/2032 | — | ||||||||||||
| 3/1/2022 | RSUs | 3/1/2023 | — | 2,094(5) | — | 208,248 | |||||||||||||
| 3/1/2022 | PSUs | — | 3,721(7) | — | 370,053 | ||||||||||||||
| 2/28/2023 | Options | 2/28/2024 | — | 20,040(5) | 138.81 | 2/28/2033 | — | ||||||||||||
| 2/28/2023 | RSUs | 2/28/2024 | — | 5,260(5) | — | 523,107 | |||||||||||||
| 2/28/2023 | PSUs | — | 5,260(7) | — | 523,107 | ||||||||||||||
| 3,209,748 | |||||||||||||||||||
| (1) | Equity awards granted prior to December 6, 2018 are subject to the terms of our 2016 Stock Option and Grant Plan, as amended from time to time (the |
| (2) | Market value reflects the value of the applicable equity award, including both vested and unvested portions, based upon the closing price for the Company’s common stock on December $99.45. |
| (3) | The shares subject to the option are fully vested. |
| (4)
| This option grant vests in three tranches. The first tranche, consisting of 50% of the underlying shares, vests as follows: 25% of this tranche vested on the first anniversary of the grant date, and the remainder vests in 12 equal quarterly installments thereafter. The second tranche, consisting of 25% of the underlying shares, vests as follows: 25% of this tranche vested on the second anniversary of the grant date, and the remainder vests in 12 equal quarterly installments thereafter. The third tranche, consisting of 25% of the underlying shares, vests as follows: 25% of this tranche vests on the third anniversary of the grant date, and the remainder will vest in 12 equal quarterly installments thereafter. Vesting is generally subject to Mr. Bancel’s continuous employment with the Company through the applicable vesting date.
|
| (5) | 25%
|
| (6) | 50% of the shares subject to the equity award vest on the second anniversary of the grant date and the remaining 50% vest in 8 equal quarterly installments thereafter, generally subject to the NEO’s continuous service relationship with the Company through each applicable vesting date.
|
| (7) | Number of PSUs assumes performance at target. The shares subject to the |
| (8) | The shares subject to the equity award vest in full on |
 | 2024 Proxy statement | 63 |
The following table sets forth the number of shares acquired and the value realized upon exercises of stock options and vesting of RSUs during the fiscal year ended December 31, 20202023 by each of our NEOs.
| Option Awards |
| Stock Awards | ||||
Number of Shares Acquired on Exercise (#) | Value Realized on Exercise ($)(1) |
| Number of Shares Acquired on Vesting (#) | Value Realized on Vesting ($) | |||
Stéphane Bancel | — | $ | — |
| — | $ | — |
David Meline | — |
| — |
| — |
| — |
Lorence Kim, M.D. | 1,895,284 |
| 88,790,995 |
| — |
| — |
Stephen Hoge, M.D. | 240,000 |
| 19,559,001 |
| — |
| — |
Tal Zaks, M.D. | 1,177,845 |
| 67,493,200 |
| — |
| — |
Juan Andres | 232,609 |
| 9,316,786 |
| — |
| — |
(1) The value realized upon the exercise of option awards is calculated as the difference between the market price of the underlying security at exercise and the exercise price of the options, exclusive of any payments or consideration provided on behalf of the NEO. | |||||||
| Option Awards | Stock Awards | ||||||||||||||
| Number of Shares Acquired on Exercise (#) | Value Realized on Exercise ($)(1) | Number of Shares Acquired on Vesting (#) | Value Realized on Vesting ($)(2) | ||||||||||||
| Stéphane Bancel | 2,027,155 | $ | 300,714,563 | — | $ | — | |||||||||
| James Mock | — | — | 5,810 | 605,751 | |||||||||||
| Stephen Hoge, M.D. | — | — | 16,291 | 1,908,414 | |||||||||||
| Arpa Garay | — | — | 6,843 | 814,837 | |||||||||||
| Shannon Thyme Klinger | — | — | 6,181 | 726,519 | |||||||||||
| (1) | The value realized upon the exercise of option awards is calculated as the difference between the market price of the underlying security at exercise and the exercise price of the options. |
| (2) | The value realized upon the vesting of stock awards is calculated based on the closing market price of a share of our common stock on the date prior to the date of vesting. |
All stock option exercises shown in the table above were conducted pursuant to a 10b5-1 plans.plan. For as long as they are executives, our NEOs are required to conduct any trades,sales of Moderna stock, including stock option exercises, pursuant to 10b5-1 plans. These plans require that the executive commit to a trading plan in advance and at a time when the Company is in an open trading window under the Company’s Insider Trading Policy.
moderna 2021 Proxy statement |49
BackThe stock option exercises shown above for Mr. Bancel were all exercises of a stock option award that was granted in August 2013 and that expired in August 2023. In May 2022, Mr. Bancel announced his intention to Contentsfully exercise the award, exercising 40,000 options each Wednesday and Thursday under a 10b5-1 plan until it was fully exercised. Mr. Bancel has committed to contributing the after-tax proceeds from the option exercise to charitable causes. Mr. Bancel’s announcement regarding the exercise of the stock option and the contribution of the proceeds to charitable causes can be found in the Current Report on Form 8-K filed by Moderna with the SEC on May 24, 2022.
 | 2024 Proxy statement | 64 |
Our Executive Severance Plan, as described above, provides for certain payments and benefits in the event of certain qualifying terminations of employment, including qualifying terminations of employment in connection with a change in control of the Company.
The table below quantifies the potential payments and benefits that would have become due to our NEOs, (other than Dr. Kim), assuming that a qualifying termination occurred on December 31, 2020.
Dr. Kim was not eligible to receive any payments or benefits in2023. In the event of termination due to death or disability, each of our named executive officers would be eligible to have any outstanding but unvested time-based equity accelerate on the same terms as other employees, up to a cap of $500 million, plus pro-rata vesting of any PSUs. In the event of an involuntary termination, on December 31, 2020 as he was no longer employed byeach of our NEOs would be eligible for accelerated vesting of 15% of any outstanding and unvested time-based equity awards, in accordance with the Company as of that date. See “Lorence Kim - Executive Retention and Separation Agreement” above for details related to payments Dr. Kim received upon departure from the Company.Company’s severance guidelines.
The closing market price of a share of our common stock on December 31, 202029, 2023 (the last trading day of 2023) was $104.47.$99.45.
| Qualifying Termination Not in Connection with a Change in Control ($)(1) |
| Qualifying Termination in Connection with a Change in Control ($)(2) |
| Qualifying Termination Not in Connection with a Change in Control ($)(1) | Qualifying Termination in Connection with a Change in Control ($)(2) | Termination Due to |
| |||||||||
Stéphane Bancel |
|
|
|
|
|
| |||||||||||
Cash Severance Payment | $ | 950,000 | (3) | $ | 1,425,000 | (4) | $ | 1,575,000 | (3) | $ | 2,362,500(4) | $ | — | ||||
Cash Incentive Bonus Payment |
| 950,000 | (5) |
| 2,375,000 | (6) | 2,362,500 | (5) | 5,906,250(6) | — | |||||||
COBRA Premiums |
| 24,312 | (7) |
| 36,468 | (8) | 26,712 | (7) | 40,069(8) | — | |||||||
Accelerated Equity Vesting (Time-Based) |
| — |
|
| 431,139,483 | (9) | |||||||||||
David Meline |
|
|
|
|
| ||||||||||||
| Accelerated Equity Vesting | 627,452 | (9) | 14,460,022(10) | 11,419,140(11) | |||||||||||||
| James Mock | |||||||||||||||||
Cash Severance Payment |
| 600,000 | (3) |
| 900,000 | (4) | 800,000 | (3) | 1,200,000(4) | — | |||||||
Cash Incentive Bonus Payment |
| 173,077 | (5) |
| 623,077 | (6) | 720,000 | (5) | 1,800,000(6) | — | |||||||
COBRA Premiums |
| 15,790 | (7) |
| 23,685 | (8) | 26,712 | (7) | 40,069(8) | — | |||||||
Accelerated Equity Vesting (Time-Based) |
| — |
|
| 12,295,452 | (9) | |||||||||||
| Accelerated Equity Vesting | 338,428 | (9) | 2,779,826(10) | 2,256,719(11) | |||||||||||||
Stephen Hoge, M.D. |
|
|
|
|
| ||||||||||||
Cash Severance Payment |
| 621,000 | (3) |
| 931,500 | (4) | 1,050,000 | (3) | 1,575,000(4) | — | |||||||
Cash Incentive Bonus Payment |
| 310,500 | (5) |
| 776,250 | (6) | 1,050,000 | (5) | 2,625,000(6) | — | |||||||
COBRA Premiums |
| 24,312 | (7) |
| 36,468 | (8) | 26,712 | (7) | 40,069(8) | — | |||||||
Accelerated Equity Vesting (Time-Based) |
| — |
|
| 125,192,466 | (9) | |||||||||||
Tal Zaks, M.D. |
|
|
|
|
| ||||||||||||
| Accelerated Equity Vesting | 455,966 | (9) | 7,306,578(10) | 5,988,865(11) | |||||||||||||
| Arpa Garay | |||||||||||||||||
Cash Severance Payment |
| 1,552,000 | (10) |
| 3,328,000 | (10) | 850,000 | (3) | 1,275,000(4) | — | |||||||
Cash Incentive Bonus Payment |
| 276,000 | (5) |
| 690,000 | (6) | 765,000 | (5) | 1,912,500(6) | — | |||||||
COBRA Premiums |
| 24,312 | (7) |
| 36,468 | (8) | 26,342 | (7) | 39,513(8) | — | |||||||
Accelerated Equity Vesting (Time-Based) |
| — |
|
| 55,188,846 | (9) | |||||||||||
Juan Andres |
|
|
|
|
| ||||||||||||
| Accelerated Equity Vesting | 248,526 | (9) | 2,180,441(10) | 1,657,334(11) | |||||||||||||
| Shannon Thyme Klinger | |||||||||||||||||
Cash Severance Payment |
| 552,000 | (3) |
| 828,000 | (4) | 800,000 | (3) | 1,200,000(4) | — | |||||||
Cash Incentive Bonus Payment |
| 276,000 | (5) |
| 690,000 | (6) | 720,000 | (5) | 1,800,000(6) | — | |||||||
COBRA Premiums |
| 24,312 | (7) |
| 36,468 | (8) | 17,341 | (7) | 26,012(8) | — | |||||||
Accelerated Equity Vesting (Time-Based) |
| — |
|
| 45,442,220 | (9) | |||||||||||
| |||||||||||||||||
| Accelerated Equity Vesting | 347,379 | (9) | 3,209,749(10) | 2,563,025(11) | |||||||||||||
moderna 2021 Proxy statement |50
 | 2024 Proxy statement | 65 |
| (1) | A “qualifying termination” means a termination other than due to cause, death or disability or a resignation for good reason and “not in connection with a change in control” means outside of the change in control period. |
| (2) | A “qualifying termination” means a termination other than due to cause, death or disability or a resignation for good reason and “in connection with a change in control” means within the change in control period. |
| (3) | Represents |
| (4) | Represents 18 months of the executive’s base salary. |
| (5) | Represents the NEO’s target annual bonus opportunity. |
| (6) | Represents 150% of the NEO’s target annual bonus opportunity plus the NEO’s target bonus opportunity, pro-rated for the number of full weeks |
| (7) | Represents 12 months of our contribution towards health insurance, based on our actual costs to provide health insurance to the NEO as of the date of termination. |
| (8) | Represents 18 months of our contribution towards health insurance, based on our actual costs to provide health insurance to the NEO as of the date of termination. |
| (9) | Represents the value of acceleration of 15% of the NEO’s unvested and outstanding time-based equity awards. The NEO would remain eligible for the pro-rata portion of any outstanding PSUs based on actual performance, but such PSUs would not be subject to accelerated vesting. |
| (10) | Represents the value of acceleration of (i) vesting of 100% of the NEO’s unvested and outstanding time-based equity awards and (ii) vesting of the NEO’s unvested and outstanding PSUs, based on the market price of a share of our common stock on December
|
| (11) | Represents the |
 | 2024 Proxy statement | 66 |
The following table provides information as of December 31, 20202023 with respect to shares of our common stock that may be issued under our existing equity compensation plans.
Plan Category | Number of Outstanding Options and Restricted Stock Units (a) |
| Weighted- Average Exercise Price of Outstanding Options (b) | Number of Securities Remaining Available for Future Issuance Under Equity Compensation Plans (Excluding Securities Reflected in Column (a)) |
|
Equity Compensation Plans Approved by Stockholders(1) | 36,251,179 | (2) | $ 17.14 | 31,552,397 | (3)(4) |
Equity Compensation Plans Not Approved by Stockholders | — |
| N/A | — |
|
TOTAL | 36,251,179 |
| $ 17.14 | 31,552,397 |
|
(1) Consists of our 2018 Stock Plan, 2016 Stock Plan, and ESPP. Following our initial public offering, we have not and will not grant any awards under our 2016 Stock Plan, but all outstanding awards under the 2016 Stock Plan will continue to be governed by their existing terms. The shares of common stock underlying any awards granted under the 2016 Stock Plan or 2018 Stock Plan that are forfeited, canceled, reacquired by us prior to vesting, satisfied without the issuance of stock, or otherwise terminated (other than by exercise) and the shares of common stock that are withheld upon exercise of a stock option or settlement of such award to cover the exercise price or tax withholding will be added to the shares of common stock available for issuance under the 2018 Stock Plan. (2) Does not include purchase rights accruing under the ESPP because the purchase right (and therefore the number of shares to be purchased) will not be determined until the end of the purchase period. (3) Consists of shares available for future issuance under the ESPP and the 2018 Stock Plan. As of December 31, 2020, 3,626,905 shares of common stock were available for issuance under the ESPP (which number includes shares subject to purchase during the current purchase period, which commenced on December 7, 2020, and the exact number of which will not be known until the end of the purchase period on May 30, 2021), and 27,925,492 shares of common stock were available for issuance under the 2018 Stock Plan. Subject to the number of shares remaining in the share reserve, under the ESPP, the maximum number of shares purchasable by any participant on any one purchase date for any purchase period, including the current period, may not exceed 3,000 shares. (4) The 2018 Stock Plan provides that the number of shares reserved and available for issuance under the plan will automatically increase each January 1, beginning on January 1, 2019, by 4% of the outstanding number of shares of our common stock on the immediately preceding December 31, or such lesser number of shares as determined by our compensation committee. The ESPP provides that the number of shares reserved and available for issuance will automatically increase each January 1, beginning on January 1, 2020, by the least of 3,240,000 shares of our common stock, 1% of the outstanding number of shares of our common stock on the immediately preceding December 31, or such lesser number of shares as determined by our compensation committee. In December 2020, our Compensation Committee determined that neither the 2018 Stock Plan nor the ESPP would increase the shares reserved thereunder for future issuances in 2021, as both plans had ample shares reserved for expected future issuances as of the end of 2020. | |||||
| Plan Category | Number of Securities to be Issued upon Exercise of Outstanding Options and Restricted Stock Units (a) | Weighted- Average Exercise Price of Outstanding Options (b) | Number of Securities Remaining Available for Future Issuance Under Equity Compensation Plans (Excluding Securities Reflected in Column (a)) | ||||||||
| Equity Compensation Plans Approved by Shareholders(1) | 30,684,628(2) | $ | 56.14 | 19,269,131(3)(4) | |||||||
| Equity Compensation Plans Not Approved by Shareholders | — | N/A | — | ||||||||
| TOTAL | 30,684,628 | $ | 56.14 | 19,269,131 | |||||||
| (1) | Consists of our 2018 Stock Plan, 2016 Stock Plan, and ESPP. Following our initial public offering, we have not and will not grant any awards under our 2016 Stock Plan, but all outstanding awards under the 2016 Stock Plan will continue to be governed by their existing terms. The shares of common stock underlying any awards granted under the 2016 Stock Plan or 2018 Stock Plan that are forfeited, canceled, reacquired by us prior to vesting, satisfied without the issuance of stock, or otherwise terminated (other than by exercise) and the shares of common stock that are withheld upon exercise of a stock option or settlement of such award to cover the exercise price or tax withholding will be added to the shares of common stock available for issuance under the 2018 Stock Plan. |
| (2) | Does not include purchase rights accruing under the ESPP because the purchase right (and therefore the number of shares to be purchased) will not be determined until the end of the purchase period. |
| (3) | Consists of shares available for future issuance under the ESPP and the 2018 Stock Plan. As of December 31, 2023, 3,172,648 shares of common stock were available for issuance under the ESPP (which number includes shares subject to purchase during the current purchase period, which commenced on December 1, 2023, and the exact number of which will not be known until the end of the purchase period on May 30, 2024), and 16,096,483 shares of common stock were available for issuance under the 2018 Stock Plan. Subject to the number of shares remaining in the share reserve, under the ESPP, the maximum number of shares purchasable by any participant on any one purchase date for any purchase period, including the current period, may not exceed 3,000 shares. |
| (4) | The 2018 Stock Plan provides that the number of shares reserved and available for issuance under the plan will automatically increase each January 1, beginning on January 1, 2019, by 4% of the outstanding number of shares of our common stock on the immediately preceding December 31, or such lesser number of shares as determined by our Compensation Committee. The ESPP provides that the number of shares reserved and available for issuance will automatically increase each January 1, beginning on January 1, 2020, by the least of 3,240,000 shares of our common stock, 1% of the outstanding number of shares of our common stock on the immediately preceding December 31, or such lesser number of shares as determined by our Compensation Committee. In December 2023, the Compensation Committee authorized an increase in the amount of shares reserved for future issuance under the 2018 Plan equivalent to 4% of our stock outstanding as of December 31, 2023, with such increase taking effect on January 1, 2024. No such increase was authorized by the Compensation Committee for the ESPP for 2024. The number does not include the increase to the 2018 Plan from January 1, 2024. |
moderna 2021 Proxy statement |51
 | 2024 Proxy statement | 67 |
We present below the ratio of annual total compensation of our median compensated employee to the annualized total compensation of Mr. Bancel, calculated in accordance with Item 402(u) of Regulation S-K.
In identifying our median employee, we reviewed the compensation of our entire employee population of approximately 1,300 U.S.5,600 global employees as of December 31, 2020, excluding all 24 of our non U.S. employees (21 employees in Switzerland and one in each of the United Kingdom, Canada and Spain).2023. In performing this analysis, we identified the median employee based on actual base pay during 2020,2023, plus all cash bonuses, and overtime pay receivedand equity awards received/granted during the year. After identifying the median employee, we then determined the value of annual equity awards received and the cash bonus for 20202023 (paid in 2021)2024), as well as other benefits such as 401(k) matching,match, in the same method used to calculate and report Mr. Bancel’s compensation.
The 20202023 total compensation for Mr. Bancel, as reported above in the 20202023 Summary Compensation Table on page 4659 was $12,855,275,$17,068,514, which reflects all elements of his compensation as determined under Item 402 of Regulation S-K. The 20202023 annual total compensation as determined under Item 402 of Regulation S-K for our median employee, who is employed in our research associate track,as a Finance Manager, was $159,114.$209,627.
The ratio of Mr. Bancel’s total compensation to our median employee’s total annual compensation for fiscal year 20202023 is 81 to 1.
The following table reports the compensation of our CEO (our Principal Executive Officer, or PEO) and the average compensation of the other Named Executive Officers (Other NEOs) as reported in the Summary Compensation Table in our proxy statements for the past four years, as well as their “compensation actually paid” (CAP), as calculated pursuant to SEC rules and certain performance measures required by the rules. The CAP values included in the table below reflect a measure of compensation which is a combined realizable and realized pay measure predicated on fair value. The grant date fair values included in the Summary Compensation Table (SCT) have been replaced with fair values reflecting the change in value of equity awards during the fiscal year. The calculations do not reflect the actual sale of stock underlying equity awards or the exercise of stock options by the executive.
| Average Compensation Actually Paid to Other NEOs(2) (e) | Value of Initial Fixed $100 Investment Based on: | Company -Selected Measure [Product Sales](4)(5) (millions) (i) | ||||||||||||||||||||||||||||||
| Year(1) (a) | Summary Compensation Table Total for PEO (b) | Compensation Actually Paid to PEO(2) (c) | Average SCT Total for Other NEOs (d) | Moderna TSR (f) | Peer Group(3) TSR (g) | Net Income (millions) (h) | ||||||||||||||||||||||||||
| 2023 | $ | 17,068,514 | $ | (155,552,174) | $ | 5,103,698 | $ | (3,939,532) | $ | 508 | $ | 115 | $ | (4,714) | $ | 6,671 | ||||||||||||||||
| 2022 | 19,363,648 | (306,219,630) | 5,822,260 | (8,967,479) | 918 | 111 | 8,362 | 18,435 | ||||||||||||||||||||||||
| 2021 | 18,155,739 | 793,044,894 | 8,256,157 | 67,035,028 | 1,298 | 125 | 12,202 | 17,675 | ||||||||||||||||||||||||
| 2020 | 12,855,275 | 469,967,605 | 5,286,409 | 54,985,594 | 534 | 126 | (747) | 200 | ||||||||||||||||||||||||
| (1) | Mr. Bancel served as our PEO in each year shown. The Other NEOs reflected in columns (d) and (e) above represent the following individuals for each of the years shown: 2023 – Arpa Garay, Stephen Hoge, Shannon Thyme Klinger and James Mock 2022 – Juan Andres, Arpa Garay, Jorge Gomez, Stephen Hoge, David Meline and James Mock 2021 – Juan Andres, Stephen Hoge, Shannon Thyme Klinger, David Meline and Corinne LeGoff 2020 – Juan Andres, Stephen Hoge, Lorence Kim, David Meline and Tal Zaks | |
 | ||
 | ||
 | ||
 | ||
| (2) | The applicable Summary Compensation Table totals reported for the PEO and the average of the Other NEOs for each year were subject to the following adjustments per Item 402(v)(2)(iii) of Regulation S-K to calculate “compensation actually paid”: |
| (3) | Peer group TSR reflects the Nasdaq Biotechnology Index for all four fiscal years disclosed, which aligns with the peer group used in our Annual Report on 10-K for each of these years. | |
| (4) | The Company has identified Product Sales from COVID-19 vaccines as the company-selected measure for the pay versus performance disclosure, as it represents the most important financial performance measure used to link compensation actually paid to the PEO and the Other NEOs in 2023 to the Company’s performance. | |
| (5) | Product Sales was chosen from the following three most important financial performance measures used by the Company to link compensation actually paid to the PEO and Other NEOs in 2023 to the Company’s performance: | |
| (1) | Mr. Bancel served as our PEO in each year shown. The Other NEOs reflected in columns (d) and (e) above represent the following individuals for each of the years shown: | |
| • | 2023 – Arpa Garay, Stephen Hoge, Shannon Thyme Klinger and James Mock | |
| • | 2022 – Juan Andres, Arpa Garay, Jorge Gomez, Stephen Hoge, David Meline and James Mock | |
| • | 2021 – Juan Andres, Stephen Hoge, Shannon Thyme Klinger, David Meline and Corinne LeGoff | |
| • | 2020 – Juan Andres, Stephen Hoge, Lorence Kim, David Meline and Tal Zaks | |
| Biographical information for each of these individuals and their positions can be found above, beginning on page 33, or in the proxy statement for the year upon which compensation was reported, under the heading “Management.” | ||
| (2) | The applicable Summary Compensation Table totals reported for the PEO and the average of the Other NEOs for each year were subject to the following adjustments per Item 402(v)(2)(iii) of Regulation S-K to calculate “compensation actually paid”: | |
 | 2024 Proxy statement | 68 |
| 2023 | 2022 | 2021 | 2020 | |||||||||||||||||||||||||||||
| PEO | Average for Other NEOs | PEO | Average for Other NEOs | PEO | Average for Other NEOs | PEO | Average for Other NEOs | |||||||||||||||||||||||||
| Summary Compensation Table | $ | 17,068,514 | $ | 5,103,698 | $ | 19,363,648 | $ | 5,822,260 | $ | 18,155,739 | $ | 8,256,157 | $ | 12,855,275 | $ | 5,286,409 | ||||||||||||||||
| Adjustments | ||||||||||||||||||||||||||||||||
| Deduction for amounts reported under the “Stock Awards” and “Option Awards” columns of the Summary Compensation Table(a) | (12,516,907) | (3,546,372) | (14,389,009) | (4,285,485) | (15,000,000) | (6,600,000) | (9,000,000) | (4,220,000) | ||||||||||||||||||||||||
| Increase/(decrease) for the Inclusion of Rule 402(v) Equity Values(a) | (160,103,781) | (5,496,858) | (311,194,269) | (10,504,254) | 789,889,155 | 65,378,871 | 466,112,330 | 53,919,185 | ||||||||||||||||||||||||
| Year-End Fair Value of Equity Awards Granted During Year That Remained Unvested as of Last Day of Year | 7,294,658 | 2,224,773 | 18,930,378 | 5,957,933 | 25,410,484 | 7,893,516 | 53,681,961 | 14,619,557 | ||||||||||||||||||||||||
| Change in Fair Value from Last Day of Prior Year to Last Day of Year of Unvested Equity Awards | (15,002,894) | (4,007,912) | (216,035,770) | (5,734,897) | 577,920,879 | 29,752,324 | 347,020,376 | 30,590,579 | ||||||||||||||||||||||||
| Change in Fair Value of Prior Years’ Equity Awards that Vested During the Year | (152,395,545) | (3,713,719) | (114,088,877) | (10,254,023) | 186,557,792 | 27,733,031 | 65,409,993 | 10,481,461 | ||||||||||||||||||||||||
| Change in Fair Value of Prior Years’ Equity Awards that Forfeited During the Year | — | — | — | (473,267) | — | — | — | (1,772,412) | ||||||||||||||||||||||||
| Compensation Actually Paid | (155,552,174) | (3,939,532) | (306,219,630) | (8,967,479) | 793,044,894 | 67,035,028 | 469,967,605 | 54,985,594 | ||||||||||||||||||||||||
| (a) | Compensation Actually Paid excludes the Stock Awards and Option Awards columns from the relevant fiscal year’s Summary Compensation Table total. The Rule 402(v) Equity Values instead reflect the aggregate of the following components, as applicable: (i) the fair value as of the end of the listed fiscal year of unvested equity awards granted in that year; (ii) the change in fair value during the listed fiscal year of equity awards granted in prior years that remained outstanding and unvested at the end of the listed fiscal year; and (iii) the change in fair value during the listed fiscal year through the vesting date of equity awards granted in prior years that vested during the listed fiscal year, less the fair value at the end of the prior year of awards granted prior to the listed fiscal year that failed to meet applicable vesting conditions during the listed fiscal year. Equity values are calculated in accordance with FASB ASC Topic 718. |
| (3) | Peer group TSR reflects the Nasdaq Biotechnology Index for all four fiscal years disclosed, which aligns with the peer group used in our Annual Report on 10-K for each of these years. | |
| (4) | The Company has identified Product Sales from COVID-19 vaccines as the company-selected measure for the pay versus performance disclosure, as it represents the most important financial performance measure used to link compensation actually paid to the PEO and the Other NEOs in 2023 to the Company’s performance. | |
| (5) | Product Sales was chosen from the following three most important financial performance measures used by the Company to link compensation actually paid to the PEO and Other NEOs in 2023 to the Company’s performance: | |
| Performance Metrics | ||
| Product Sales from COVID-19 Vaccines | Refer to “Compensation Discussion and Analysis—Short-Term Incentive Compensation—2023 Corporate Objectives,” described on page 48. | |
| Operating Income | Refer to “Compensation Discussion and Analysis—Short-Term Incentive Compensation—2023 Corporate Objectives,” described on page 48. | |
| Total Shareholder Return (TSR) | Stock options are the most heavily weighted equity vehicle in our long-term incentive program (75% for the PEO and 50% for the Other NEOs). The value of these equity awards is directly related to stock price appreciation over time, which incentivizes our executives to achieve our long-term strategic and pipeline goals to create shareholder value. Total Shareholder Return on a $100 investment in Moderna stock over a four-year period ending December 31, 2023 would have been $508.44. | |
 | 2024 Proxy statement | 69 |
| • | Description of the relationship between Moderna’s TSR and peer group TSR |
| • | Descriptions of the relationships between Compensation Actually Paid (CAP) and financial measures for the PEO and the average of the Other NEOs |

TSR for our peer group is based on the Nasdaq Biotechnology Index, which reflects the Company’s industry sector and is also the peer group used in our Annual Report on Form 10-K.
Moderna significantly outperformed peers in 2021 and 2022, reflecting our rapid shift to a commercial company as we developed one of the earliest COVID-19 vaccines during the pandemic. The COVID-19 vaccine was Moderna’s first commercial product when it was authorized for emergency use in the U.S. in December 2020, followed quickly by authorization or approval in markets globally.
Our stock price declined at the end of 2023 compared to the prior year primarily driven by the lower demand for COVID-19 vaccines as we entered the endemic market. We anticipate that the advancement of our pipeline, with as many as 15 products in the next five years, will build significant value for our investors, which should be reflected in our stock price.

Equity awards are the largest component of our executive compensation program, representing no less than 70% of the target total compensation for each of our executives. Therefore, TSR has a significant impact on our CAP. The impact for our CEO/PEO is more pronounced given the heavier weighting of stock options versus the other NEOs (75% vs. 50%) and the larger size of his annual equity awards.
Prior to our development of the COVID-19 vaccine early in 2020, two members of our current executive team (Mr. Bancel and Dr. Hoge) were granted equity awards at then-prevailing stock prices. From the time of our IPO in December 2018 through early February 2020—when we completed the first batch of our COVID-19 vaccine for testing—our stock price fluctuated between a low of $11.54 and a high of $29.79. Equity awards granted prior to the development of our COVID-19 vaccine increased significantly in value during 2021, a year in which our closing stock price on December 31, 2021 was $253.98.
Our Other NEOs joined Moderna in 2021 and 2022 (Mr. Mock and Mses. Garay and Klinger) and were granted equity awards at higher stock prices and therefore, the impact of stock price fluctuations on their CAP has been less pronounced. In addition, as of December 31, 2023, all of their previously granted stock options are underwater, with grant prices significantly above prevailing stock prices. The decline in our stock price during 2022 and 2023 has resulted in negative CAP for our PEO and Other NEOs.
 | 2024 Proxy statement | 70 |

Our stock price appreciated significantly during 2020 as we announced positive milestones in connection with the development of our COVID-19 vaccine and in anticipation of our delivering on the promise of mRNA medicine. COVID-19 vaccines were the first medicine using mRNA technology, and prior to their launch many questioned the ability to develop medicines using mRNA. While we recognized our initial sales to the U.S. and Canadian governments in the last few weeks of 2020, we incurred significant costs during the year to produce the vaccine and to prepare for global distribution. As a result, we experienced a loss in 2020, but experienced a significant growth in net income for 2021. 2022 also saw positive net income, but a decline from the prior year. Moderna experienced a loss in 2023 driven by lower sales of our COVID-19 vaccine, as further described in our Annual Report on Form 10-K.

The most heavily weighted factor in Moderna’s corporate scorecard with respect to Moderna’s annual bonus plan is our product sales. Product sales from our COVID-19 vaccine are our primary source of revenue and are key to funding our operations and ongoing investments in the rest of our research and development pipeline. Most of our other scorecard goals are tied to advancing our product pipeline.
Our product sales increased significantly during 2021 with the sale of our first commercial product, the COVID vaccine, during the global pandemic. Our product sales increased by an additional 4% between 2021 and 2022, but declined in 2023 as we moved into an endemic market and experienced lower than anticipated vaccination rates. We believe that our ongoing investments in advancing our pipeline will lead to future revenues, and that this will in turn lead to shareholder value creation that will be reflected in our stock price.
 | 2024 Proxy statement | 71 |
Tax and Accounting Considerations
Deductibility of Executive Compensation
Generally, Section 162(m) of the Code (“Section(Section 162(m)”) disallows a federal income tax deduction for public corporations of remuneration in excess of $1 million paid in any fiscal year to certain specified executive officers. Subject to certain transition rules which apply to remuneration provided pursuant to written binding contracts which were in effect on November 2, 2017, and which are not subsequently modified in any material respect and certain transition relief for newly public companies, for taxable years beginning after December 31, 2017, the exemption from the deduction limit for “performance-based compensation” is no longer available. Consequently, for fiscal years beginning after December 31, 2017, all remuneration in excess of $1 million paid to a specified executive will not be deductible unless it qualifies for the transition relief described above.
In designing our executive compensation program and determining the compensation of our executive officers, including our NEOs, the Compensation Committee considers a variety of factors, including the potential impact of the Section 162(m) deduction limit. However, the Compensation Committee will not necessarily limit executive compensation to that which is or may be deductible under Section 162(m). The deductibility of some types of compensation depends upon the timing of an executive officer’s vesting or exercise of previously granted rights. Further, interpretations of and changes in the tax laws, and other factors beyond the Compensation Committee’s control also affect the deductibility of compensation. The Compensation Committee will consider various alternatives to preserving the deductibility of compensation payments and benefits to the extent consistent with its compensation goals.
To maintain flexibility to compensate our executive officers in a manner designed to promote our short-term and long-term corporate goals, the Compensation Committee has not adopted a policy that all compensation must be deductible. The Compensation Committee believes that our stockholders’shareholders’ interests are best served if its discretion and flexibility in awarding compensation is not restricted in order to allow such compensation to be consistent with the goals of our executive compensation program, even though some compensation awards may result in non-deductible compensation expense.
We follow the Financial Accounting Standard Board’s Accounting Standards Codification Topic 718 (“FASB(FASB ASC Topic 718”)718) for our stock-based compensation awards. FASB ASC Topic 718 requires us to measure the compensation expense for all share-based payment awards made to our employees and non-employee members of our Board of Directors, including stock options to purchase shares of our common stock and other stock awards, based on the grant date “fair value” of these awards. This calculation is performed for accounting purposes and reported in the executive compensation tables required by the federal securities laws, even though the recipient of the awards may never realize any value from their awards.
moderna 2021 Proxy statement |52
Taxation of “Parachute” Payments
Sections 280G and 4999 of the Code provide that executive officers and directors who hold significant equity interests and certain other service providers may be subject to significant additional taxes if they receive payments or benefits in connection with a change in control of the company that exceeds certain prescribed limits, and that the company (or a successor) may forfeit a deduction on the amounts subject to this additional tax. We have not agreed to provide any executive officer, including any NEO, with a “gross-up” or other reimbursement payment for any tax liability that the executive officer might owe as a result of the application of Sections 280G or 4999 of the Code.
Section 409A of the Code imposes additional significant taxes in the event that an executive officer, director or service provider receives “deferred compensation” that does not satisfy the requirements of Section 409A of the Code. Although we do not maintain a traditional nonqualified deferred compensation plan, Section 409A of the Code does apply to certain severance arrangements, bonus arrangements and equity awards. We structure all our severance arrangements, bonus arrangements and equity awards in a manner to either avoid the application of Section 409A or, to the extent doing so is not possible, to comply with the applicable requirements of Section 409A of the Code.
 | 2024 Proxy statement | 72 |
The information contained in this Compensation and Talent Committee Report shall not be deemed to be soliciting material or to be filed with the Securities and Exchange Commission, subject to Regulations 14A or 14C of the Securities Exchange Act of 1934, as amended (the “Exchange Act”)Exchange Act), or subject to the liabilities of Section 18 of the Exchange Act. No portion of this Compensation and Talent Committee Report shall be deemed to be incorporated by reference into any filing under the Securities Act of 1933, as amended (the “Securities Act”)Securities Act) or the Exchange Act, through any general statement incorporating by reference in its entirety the proxy statement in which this report appears, except to the extent that Moderna specifically incorporates this report or a portion of it by reference. In addition, this report shall not be deemed filed under either the Securities Act or the Exchange Act.
The Compensation and Talent Committee has reviewed and discussed the section captioned “Compensation Discussion and Analysis” with management. Based on such review and discussions, the compensationCompensation and talent committeeTalent Committee recommended to the Board of Directors that this “Compensation Discussion and Analysis” section be included in this proxy statement.
Respectfully submitted by the members of the Compensation and Talent Committee of the Board of Directors:
Stephen Berenson
François Nader (Chairperson)
Francois Nader
Paul Sagan
Elizabeth Nabel
Elizabeth Tallett
moderna 2021 Proxy statement |53
 | 2024 Proxy statement | 73 |
The following table sets forth certain information known to us regarding beneficial ownership of our common stock as of March 9, 2021,7, 2024, or as of the date otherwise set forth below, for:
each person or group of affiliated persons known by us to be the beneficial owner of more than five percent of our capital stock;
| • | each person or group of affiliated persons known by us to be the beneficial owner of more than five percent of our capital stock; |
| • | each of our named executive officers; |
| • | each of our directors; and |
| • | all of our executive officers, directors, and director nominees as a group. |
each of our named executive officers;
each of our directors;
each of our director nominees; and
all of our executive officers, directors, and director nominees as a group.
Beneficial ownership is determined in accordance with the rules of the SEC and generally includes voting or investment power with respect to securities. Under those rules, beneficial ownership includes any shares as to which the individual or entity has sole or shared voting power or investment power. Except as noted by footnote, and subject to community property laws where applicable, we believe, based on the information provided to us, that the persons and entities named in the table below have sole voting and investment power with respect to all common stock shown as beneficially owned by them.
The percentage of beneficial ownership in the table below is based on 400,525,387382,879,612 shares of common stock outstanding as of March 9, 2021.7, 2024. The table shown below and the calculated percentage of beneficial ownership includes shares owned by each stockholdershareholder and all stock options held by such stockholdershareholder that are either currently vested or will be vested within 60 days of March 9, 2021.7, 2024. Further details are provided in the footnotes section below the table.
| Shares Beneficially Owned | ||
Name and Address of Beneficial Owner(1) | Number | Percentage |
|
Named Executive Officers, Directors and Director Nominees: |
|
| |
Stéphane Bancel, Chief Executive Officer and Director(2) | 31,767,237 | 7.9 | % |
Noubar B. Afeyan, Ph.D., Chairman(3) (see Flagship Pioneering and affiliated entities below) | 26,624,078 | 6.6 | % |
Robert Langer, Sc.D., Director(4) | 11,793,363 | 2.9 | % |
Stephen Hoge M. D., President(5) | 5,283,176 | 1.3 | % |
Juan Andres, Chief Technical Operations and Quality Officer(6) | 791,556 | * | % |
Paul Sagan, Director(7) | 623,749 | * | % |
Stephen Berenson, Director(8) | 178,236 | * | % |
Tal Zaks, M.D., Chief Medical Officer(9) | 80,183 | * | % |
Francois Nader, M.D., Director(10) | 75,879 | * | % |
Lorence Kim, M.D., Former Chief Financial Officer(11) | 65,000 | * | % |
Sandra Horning, M.D., Director(12) | 35,466 | * | % |
Elizabeth Tallett, Director(13) | 11,229 | * | % |
David Meline, Chief Financial Officer(14) | 1,500 | * | % |
Elizabeth Nabel, M.D., Director(15) | 0 | * | % |
All executive officers, directors and director nominees as a group (16 persons)(16) | 77,679,823 | 19.4 | % |
Other 5% Stockholders: |
|
|
|
Baillie Gifford and Co.(17) | 44,691,386 | 11.2 | % |
Flagship Pioneering and affiliated entities(18) (see Noubar B. Afeyan, Ph.D., Chairman above) | 24,366,937 | 6.1 | % |
The Vanguard Group(19) | 23,371,855 | 5.8 | % |
BlackRock, Inc.(20) | 20,476,714 | 5.1 | % |
* Represents beneficial ownership of less than one percent |
|
|
|
| Shares Beneficially Owned | ||||
| Name and Address of Beneficial Owner(1) | Number | Percentage | ||
| Named Executive Officers, Directors and Director Nominees: | ||||
| Stéphane Bancel, Chief Executive Officer and Director(2) | 30,113,590 | 7.7% | ||
| Noubar B. Afeyan, Ph.D., Chairman(3) | 12,017,153 | 3.1% | ||
| Robert Langer, Sc.D., Director(4) | 11,808,993 | 3.1% | ||
| Stephen Hoge, M.D., President(5) | 5,312,014 | 1.4% | ||
| Paul Sagan, Director(6) | 587,832 | * | ||
| Stephen Berenson, Director(7) | 190,384 | * | ||
| François Nader, M.D., Director(8) | 89,016 | * | ||
| Shannon Thyme Klinger, Chief Legal Officer(9) | 51,355 | * | ||
| Sandra Horning, M.D., Director(10) | 51,114 | * | ||
| Elizabeth Tallett, Director(11) | 40,152 | * | ||
| James Mock, Chief Financial Officer(12) | 27,216 | * | ||
| Arpa Garay, Former Chief Commercial Officer(13) | 25,150 | * | ||
| Elizabeth Nabel, M.D., Director(14) | 15,987 | * | ||
| All executive officers, directors and director nominees as a group (13 persons) | 60,329,956 | 15.2% | ||
| Other 5% Shareholders: | ||||
| Baillie Gifford & Co(15) | 45,654,527 | 11.9% | ||
| The Vanguard Group(16) | 33,906,974 | 8.9% | ||
| BlackRock, Inc.(17) | 25,301,057 | 6.6% | ||
| * | Represents beneficial ownership of less than one percent |
moderna 2021 Proxy statement |54
 | 2024 Proxy statement | 74 |
| (1) | Unless otherwise indicated, the address for each beneficial owner is c/o Moderna, Inc., 200 Technology Square, Cambridge, MA 02139. | |
| (2) | The shares reported herein consist of (a) | |
| (3) | Consists of
Funds and Flagship Pioneering. | |
| (4) | Consists of (a) 11,466,961 shares held by Robert Langer, (b) 14,132 shares held by Michael D. Langer Irrevocable Trust u/d/t dated 12/14/95, (c) 14,132 shares held by Susan K. Langer Irrevocable Trust u/d/t dated 12/14/95, (d) 14,132 shares held by Samuel A. Langer Irrevocable Trust u/d/t dated 12/14/95, and (e) 7, 2024. | |
| (5) | Consists of (a) | |
| (6) | Consists of (a) 703 shares held, by Paul Sagan (b) 370,407 shares held by Paul Sagan Revocable Trust, (c)
7, 2024. | |
| (7) | Consists of (a)
7, 2024. | |
| (8) | Consists of 7, 2024. | |
| (9) | Consists of 7, 2024. | |
| (10) | Consists of
7, 2024. | |
| (11) | Consists of
7, 2024. | |
| (12) | Consists of (a) 7, 2024. | |
| (13) | Consists of (a) 4,931 shares held and (b) 20,219 shares of common stock underlying outstanding stock options or unvested RSUs held by Arpa Garay that are or will be immediately exercisable or vest within 60 days of March 7, 2024. | |
| (14) | Consists of (a) 1,373 shares held and (b) 14,614 shares of common stock underlying outstanding stock options or unvested RSUs held by Elizabeth Nabel that are or will be immediately exercisable or vest within 60 days of March 7, 2024. | |
| (15) | Based solely on a Schedule 13G/A filed | |
| (16) | Based solely on a Schedule 13G/A filed February
| |
| (17) | Based solely on a Schedule |
 | 2024 Proxy statement | 75 |
Section 16(a) Beneficial Ownership Reporting Compliance
Section 16(a) of the Exchange Act requires our directors and executive officers and persons who own more than 10% of a registered class of our equity securities to file with the SEC initial reports of beneficial ownership and reports of changes in beneficial ownership, and to furnish us with copies of all such reports. To our knowledge, based solely on a review of the copies of such reports furnished to us, or written representation that no other reports were required, we believe that for the year ended December 31, 2020, all required reports were filed on a timely basis under Section 16(a) of the Exchange Act, except that due to an administrative oversight, a Form 4 was not filed reflecting the new hire equity award for David Meline that was issued on July 6, 2020 until January 11, 2021.
moderna 2021 Proxy statement |55
Our Audit Committee has appointed Ernst & Young LLP as our independent registered public accounting firm to audit our consolidated financial statements for the year ending December 31, 2021.2024. Ernst & Young has served as our independent registered public accounting firm since 2014. As a matter of good corporate governance, we are asking stockholdersshareholders to ratify this appointment. If this appointment is not ratified at the Annual Meeting, the Audit Committee may reevaluate the selection of our auditors, but is not required to do so. Even if stockholdersshareholders ratify this appointment, the Audit Committee, in its sole discretion, may appoint another independent registered public accounting firm at any time if the committee believes that such a change would be in the best interests of Moderna and its stockholders.shareholders.
A representative of Ernst & Young is expected to attend the Annual Meeting, will have an opportunity to make a statement if he or she wishes to do so, and will be available to respond to appropriate questions from stockholders.shareholders.
During 2023, our organization continued to grow geographically, and a number of our foreign subsidiaries were subject to new audit requirements as they matured. Additionally, the shift to a commercial market in the U.S. significantly increased the number of our customers and this, as well as other factors related to the growth of our business, as well as a reduced materiality threshold, increased the complexity of our annual audit. As a result, we experienced an increase in our year-over-year audit fees from Ernst & Young.
Our Audit Committee remains focused on ensuring the independence of Ernst & Young. In 2023, we significantly reduced spending on tax fees versus 2022 as we continued to hire internal talent and other service providers to perform certain tax-related services for which we had previously engaged Ernst & Young. As we have disclosed in prior years, Ernst & Young was engaged to perform certain tax-related services on a temporary basis during our rapid expansion into new jurisdictions during the pandemic, but over the last two years we have been focused on limiting these engagements.
The following table presents fees for professional audit services and other services rendered to us by Ernst & Young for the years ended December 31, 20202023 and December 31, 2019:2022:
| 2023 | 2022 | ||||||
| Audit fees(1) | $ | 5,271,731 | $ | 3,733,808 | |||
| Audit-related fees(2) | — | — | |||||
| Tax fees(3) | 115,872 | 1,198,000 | |||||
| All other fees(4) | 23,464 | 6,325 | |||||
| Total Fees | $ | 5,411,067 | $ | 4,938,133 | |||
| (1) | Audit fees in 2023 and 2022 include fees for our annual audit, quarterly review procedures, statutory audits, comfort letters and consents, and assistance with and review of documents filed with the SEC. |
| (2) | There were no audit-related fees incurred in 2023 or 2022. |
| (3) | Tax fees include tax advisory and transfer pricing services. As of 2023, Ernst & Young no longer provides tax return services to Moderna. |
| (4) | All other fees in 2023 and 2022 consisted of subscription fees for Ernst & Young’s online accounting research tool, and equal pay analysis for Moderna Switzerland. |
 | 2024 Proxy statement | 76 |
|
| 2020 |
| 2019 |
Audit fees(1) | $ | 2,107,500 | $ | 1,711,806 |
Audit-related fees(2) |
| — |
| — |
Tax fees(3) |
| 1,986,000 |
| 296,686 |
All other fees(4) |
| 5,005 |
| — |
Total Fees | $ | 4,098,505 | $ | 2,008,492 |
(1) Audit fees in 2019 and 2020 include fees for our annual audit, quarterly review procedures, comfort letters and consents, assistance with and review of documents filed with the SEC, and other fees in connection with our adoption of new accounting pronouncements. (2) There were no audit-related fees incurred in 2019 or 2020. (3) Tax fees relate to tax return preparation and tax advisory services, and increased in 2020 compared to 2019 due to the Company’s commercialization and international expansion, and tax services provided in connection with those activities. (4) All other fees in 2020 consisted of subscription fees for Ernst & Young’s online accounting research tool. | ||||
During 20202023 and 2019,2022, all audit and non-audit services by our independent registered public accounting firm were pre-approved by our Audit Committee. The Audit Committee may establish pre-approval policies and procedures, subject to SEC and Nasdaq rules and regulations, for such services. The Audit Committee may delegate authority to pre-approve non-audit services to one or more members of the committee. Any decision to pre-approve an activity must be reported to the full Audit Committee at its first meeting following such decision.
In 2020,2023, Ernst & Young did not provide any services to Moderna that would have caused our Audit Committee to reconsider that firm’s independence.
The ratification of the appointment of Ernst & Young requires the affirmative vote of a majority of the shares of our common stock present in person or by proxy at the Annual Meeting and entitled to vote thereon.votes properly cast. Abstentions will have no effect on the effectresults of a vote AGAINST the proposal.this vote.
  | The Board of Directors recommends a vote “FOR” the ratification of the appointment of Ernst & Young LLP as our independent registered public accounting firm for the year ending December 31, |
 | 2024 Proxy statement | 77 |
moderna 2021 Proxy statement |56
The information contained in the following Audit Committee Report shall not be deemed to be soliciting material or to be filed with the Securities and Exchange Commission, nor shall such information be incorporated by reference into any future filing under the Securities Act of 1933, as amended, or the Securities Exchange Act of 1934, as amended, except to the extent that Moderna specifically incorporates it by reference in such filing.
The Audit Committee serves as the representative of Moderna’s Board of Directors with respect to its oversight of:
Moderna’s accounting and financial reporting processes and the audit of our financial statements;
| • | Moderna’s accounting and financial reporting processes and the audit of Moderna’s financial statements; |
| • | the integrity of Moderna’s financial statements; |
| • | Moderna’s compliance with legal and regulatory requirements; |
| • | significant risks, including cybersecurity risks, reviewing Moderna’s policies for risk assessment and risk management, and assessing the steps management has taken to control these risks; |
| • | the performance and responsibilities of Moderna’s internal audit function; and |
| • | the appointment, qualifications, and independence of the independent registered public accounting firm. |
the integrity of Moderna’s financial statements;
Moderna’s compliance with legal and regulatory requirements;
significant risks, reviewing Moderna’s policies for risk assessment and risk management, and assessing the steps management has taken to control these risks; and
the appointment, qualifications, and independence of the independent registered public accounting firm.
The Audit Committee also reviews the performance of the independent registered public accounting firm in the annual audit of Moderna’s financial statements and in assignments unrelated to the audit, and reviews the independent registered public accounting firm’s fees.
The Audit Committee is composed of three non-employee directors. The Board of Directors has determined that each member of the Audit Committee is independent and that Ms. Tallett, Mr. Berenson and Mr. Sagan each qualifies as an “audit committee financial expert” under SEC rules.
The Audit Committee provides the Board of Directors such information and materials as it may deem necessary to apprise the Board of Directors of financial matters requiring its attention. The Audit Committee reviews Moderna’s financial disclosures and meets privately, outside the presence of management, with the independent registered public accounting firm.firm and Moderna’s internal auditors. In fulfilling its oversight responsibilities, the Audit Committee reviewed and discussed the audited financial statements in Moderna’s Annual Report on Form 10-K for the year ended December 31, 2020,2023, with management, Moderna’s internal auditors and with Ernst & Young, including a discussion of the quality and substance of the accounting principles, the reasonableness of significant judgments made in connection with the audited financial statements, and the clarity of disclosures in the financial statements. The Audit Committee reports on these meetings to the Board of Directors.
The Audit Committee has discussed with Ernst & Young the matters required to be discussed by the applicable requirements of the Public Company Accounting Oversight Board (“PCAOB”)(PCAOB) and the SEC. In addition, the Audit Committee has received and reviewed the written disclosures and the letter from Ernst & Young required by the applicable requirements of the PCAOB regarding that firm’s communications with the Audit Committee concerning independence, and has discussed with Ernst & Young its independence from management and from Moderna, and considered the compatibility of non-audit services with Ernst & Young’s independence. In addition, the Audit Committee discussed Moderna’s internal controls over financial reporting and management’s assessment of the effectiveness of those controls with management, Moderna’s internal auditors and Ernst & Young. The Audit Committee reviewed with both Ernst & Young and Moderna’s internal auditors their audit plans, audit scope and identification of audit risks. Based on these reviews and discussions, the Audit Committee recommended to the Board of Directors that Moderna’s audited consolidated financial statements be included in Moderna’s Annual Report on Form 10-K for the year ended December 31, 2020,2024, for filing with the SEC.
The Audit Committee has selected Ernst & Young as Moderna’s independent registered public accounting firm for the year ending December 31, 2021.2024. The Board of Directors recommends that stockholdersshareholders ratify this selection at the Annual Meeting.
Respectfully submitted by the members of the Audit Committee of the Board of Directors:
Elizabeth Tallett (Chairperson)
Stephen Berenson
Paul Sagan
moderna 2021 Proxy statement |57
 | 2024 Proxy statement | 78 |
This management proposal seeks to provide to common shareholders owning at least 20% of Moderna’s outstanding stock the right to call a special meeting of shareholders, in accordance with, and subject to, the provisions that would be set forth in our governing documents.
As part of our continuous evaluation of our corporate governance practices, our Board regularly reviews our governing documents and considers feedback from investors concerning possible updates. Currently, our Amended and Restated Certificate of Incorporation (the “Certificate of Incorporation”) provides that only the Board may call a special meeting of shareholders.
In our engagement meetings with shareholders, we have received consistent feedback that shareholders would appreciate an expansion of certain shareholder rights. This feedback and engagement with shareholders informed the Board’s decision to amend our By-laws in February 2024 to implement majority voting for uncontested director elections, as well as proxy access on market standard terms, both as described elsewhere in this Proxy Statement. Additionally, the Board has decided that it is in the best interests of the Company and our shareholders to permit shareholders that collectively hold a sufficiently large economic and voting interest in the Company to require that the Company call a special meeting of shareholders, subject to specified procedures, provisions and requirements.
The Board, based on the recommendation of the Nominating and Corporate Governance Committee, has unanimously recommended that the Company’s shareholders vote “FOR” an amendment (the “Special Meeting Amendment”) to the Certificate of Incorporation to amend and revise Article V, Section 2 to read in its entirety as follows:
“2. Special Meetings. Except as otherwise required by statute and subject to the rights, if any, of the holders of any series of Undesignated Preferred Stock, special meetings of the stockholders of the Corporation may be called only by (i) the Board of Directors acting pursuant to a resolution approved by the affirmative vote of a majority of the Directors then in office (whether or not there exist any vacancies in previously authorized directorships at the time any such resolution is presented to the Board of Directors for adoption), (ii) the chairman of the Board of Directors, or the chief executive officer or president (in the absence of a chief executive officer) or (iii) the secretary of the Corporation following the secretary’s receipt of signed written requests to call a meeting from the holders of at least 20% of the voting power of the capital stock of the Corporation issued and outstanding and entitled to vote. Except as otherwise required by law, special meetings of stockholders of the Corporation may not be called by any other person or persons. Advance notice of stockholder nominations and business to be brought before any special meeting of the stockholders of the Corporation shall be given in the manner provided in the By-laws of the Corporation.”
If the shareholders approve the Special Meeting Amendment at the Annual Meeting, the Company will file a Certificate of Amendment to our Certificate of Incorporation in the form attached hereto as Appendix A. In accordance with Delaware law, however, our Board may elect to abandon the Special Meeting Amendment without further action by the shareholders at any time prior to the effectiveness of the filing of the Special Meeting Amendment with the Secretary of State of the State of Delaware, notwithstanding shareholder approval of the amendment.
The Board recognizes that providing a significant portion of the shareholders of a company the ability to call special meetings is viewed by many shareholders as an important shareholder right. However, the Board also recognizes the need for appropriate parameters given that special meetings of shareholders potentially can be disruptive to business operations and to long-term shareholder interests, can be misused and can cause the Company to incur substantial expenses. Accordingly, the Board believes that the proposed 20% threshold for calling special meetings of shareholders will balance these considerations, providing that special meetings can be called by shareholders with a significant interest in the Company, while minimizing the risk of disruption to the Company and its operations that calling a special meeting could entail. The Board’s decision to set this threshold at 20% was informed by feedback from investors during outreach meetings over the last several months, and the Board believes this level of support strikes an appropriate balance based upon the views expressed during those meetings.
 | 2024 Proxy statement | 79 |
The Board further believes that the Special Meeting Amendment will further align the Company’s governance with prevailing market practices by granting the right call a special meeting of shareholders to the Chairman of the Board, the Chief Executive Officer, or the President (in the absence of a Chief Executive Officer).
As described above, the Special Meeting Amendment would amend our Certificate of Incorporation to permit shareholders holding at least 20% of our outstanding stock to request that the Secretary of the Company call a special meeting of shareholders. It would also grant the Chairman of the Board, the Chief Executive Officer, or the President (in the absence of a Chief Executive Officer), the right to call a special meeting of shareholders. Any business to be acted upon at a special meeting of shareholders would be subject to the applicable provisions of our By-laws.
If the Special Meeting Amendment is approved, the Board will adopt corresponding changes to the By-laws (which do not require shareholder approval) that will include safeguards and requirements for calling special meetings, including the concepts outlined below and otherwise consistent with the newly-effective shareholder rights provided in the Certificate of Incorporation:
| • | To specify the information required to be set forth in a written request to call a special meeting; |
| • | To specify the requirements for proponents to update and supplement information submitted in connection with a special meeting to ensure that information remains accurate in advance of the meeting; |
| • | To grant the Board the discretion to determine whether to hold a special meeting if written requests to hold a special meeting are revoked from holders and the relevant threshold is no longer met; |
| • | To grant the Board the right to determine the place (if any), date and time of a special meeting and to set the record date for the special meeting, as well as to determine any other business to be transacted at the special meeting; and |
| • | To specify that a shareholder written request to call a special meeting shall not be valid if it (i) relates to an item of business that is not a proper subject for shareholder action under applicable law, (ii) relates to an item that is the same or substantially similar to an item that was voted upon at a shareholder meeting within 120 days prior to the receipt of shareholder requests, (iii) that is delivered during the period commencing 120 days prior to the anniversary of the last annual meeting and ending on the date of the next annual meeting, (iv) violates Regulation 14A under the Exchange Act, or (v) does not otherwise comply with the by-law related to special meetings. |
Other than the revisions to Article V, Section 2 to reflect the text that appears above, the Special Meeting Amendment would not cause any changes to the Certificate of Incorporation. If the Special Meeting Amendment is approved by shareholders, the Special Meeting Amendment would become effective upon filing with the Delaware Secretary of State, which the Company would submit promptly following the Annual Meeting of Shareholders.
 | The Board of Directors recommends a vote “FOR” this proposal, for the reasons outlined above. |
 | 2024 Proxy statement | 80 |
In August 2022, the State of Delaware, which is the Company’s state of incorporation, enacted legislation that enables Delaware companies to limit the liability of certain officers in limited circumstances. The amended Delaware law permits exculpation for direct claims brought by shareholders for breach of an officer’s fiduciary duty of care, but would not permit companies to eliminate officers’ monetary liability for breach of fiduciary duty claims brought by the company itself or for derivative claims brought by shareholders on behalf of the company. The new law also does not allow officers to be exculpated for breaches of the duty of loyalty, acts or omissions not in good faith or that involve intentional misconduct or a knowing violation of law, or any transaction in which an officer derived an improper personal benefit.
In light of this change in law, the Board, based on the recommendation of the Nominating and Corporate Governance Committee, has unanimously recommended that the Company’s shareholders vote “FOR” an amendment (the “Exculpation Amendment”) to the Company’s Amended and Restated Certificate of Incorporation (the “Certificate of Incorporation”) to add a new Article VIII, which shall read in its entirety as follows:
“ARTICLE VIII. Limitation of Liability for Officers. An Officer (as defined below) of the Corporation shall not be personally liable to the Corporation or its stockholders for monetary damages for breach of his or her fiduciary duty as an officer of the Corporation, except for liability (a) for any breach of the Officer’s duty of loyalty to the Corporation or its stockholders, (b) for acts or omissions not in good faith or which involve intentional misconduct or a knowing violation of law, (c) for any transaction from which the Officer derived an improper personal benefit, or (d) arising from any claim brought by or in the right of the Corporation. If the DGCL is amended after the effective date of this Certificate to authorize corporate action further eliminating or limiting the personal liability of Officers, then the liability of an Officer of the Corporation shall be eliminated or limited to the fullest extent permitted by the DGCL, as so amended. For purposes of this Article VIII, “Officer” shall mean an individual who has been duly appointed as an officer of the Corporation and who, at the time of an act or omission as to which liability is asserted, is deemed to have consented to service by the delivery of process to the registered agent of the Corporation as contemplated by 10 Del. C. § 3114(b).
Any amendment, repeal or modification of this Article VIII by either of (i) the stockholders of the Corporation or (ii) an amendment to the DGCL, shall not adversely affect any right or protection existing at the time of such amendment, repeal or modification with respect to any acts or omissions occurring before such amendment, repeal or modification of a person serving as a Director at the time of such amendment, repeal or modification.”
The Exculpation Amendment also includes a corresponding renumbering of the subsequent Articles in the Certificate of Incorporation and a technical amendment in the (newly-renumbered) Article IX to clarify that the Board may adopt (in addition to amend or repeal) the Bylaws.
If the shareholders approve this proposal at the Annual Meeting, the Company will file a Certificate of Amendment to our Certificate of Incorporation substantially in the form attached hereto as Appendix B. In accordance with Delaware law, however, our Board may elect to abandon the Exculpation Amendment without further action by the shareholders at any time prior to the effectiveness of the filing of the Exculpation Amendment with the Secretary of State of the State of Delaware notwithstanding shareholder approval of the amendment.
The Board believes it is important to provide protection from certain liabilities and expenses that may discourage prospective or current officers from serving the Company. The Board believes it is appropriate for public companies in states that allow exculpation of officers to have exculpation clauses in their company charters. In the absence of such protection, qualified officers might be deterred from serving as officers due to exposure to personal liability and the risk that substantial expense will be incurred. Our Board and our shareholders expect our officers to effectively manage the Company’s challenges within a complex and ever-evolving environment. The Board recognizes that our officers are called upon to make critical decisions, and that in an increasingly litigious environment our executive team can be exposed to substantial personal liability from litigants seeking to impose liability on the basis of hindsight and regardless of merit. The Board believes that providing the protection permitted under Delaware law will provide comfort to and empower our officers to best exercise their business judgment in the interests of the Company and our shareholders, which
 | 2024 Proxy statement | 81 |
will encourage agility and critical decision-making, while also enabling us to continue to attract and retain experienced and high-quality officers.
To the extent our peers adopt similar exculpation clauses that limit the personal liability of officers, failing to adopt the Exculpation Amendment could negatively impact our recruitment of exceptional officer candidates and retention of current officers.
Our Certificate of Incorporation currently provides for the exculpation of directors but does not include a provision that allows for the exculpation of officers. The Exculpation Amendment would more generally align the protections available to our directors under our governing documents and Delaware law with those available to our officers, enabling them to exercise their business judgment in furtherance of the interests of the shareholders without the potential for distraction posed by the risk of personal liability. The Exculpation Amendment would also help to clarify the application of exculpation provisions to individuals serving as both a director and an officer. As is currently the case with directors under our Certificate of Incorporation, this provision would not exculpate officers from liability for breach of the duty of loyalty, acts or omissions not in good faith or that involve intentional misconduct or a knowing violation of law, or any transaction in which the officer derived an improper personal benefit. Nor would this provision exculpate such officers from liability for claims brought by or in the right of the Company, such as derivative claims.
The Exculpation Amendment is aimed at striking a balance between shareholders’ interest in accountability and their interest in the Company being able to attract and retain experienced and high-quality officers to work on its behalf. Accordingly, the Board believes that the Exculpation Amendment is in the best interests of the Company and its shareholders.
Given the narrow class and type of claims for which officers’ liability would be exculpated, the Board believes the Exculpation Amendment would not negatively impact shareholder rights.
The Exculpation Amendment may also reduce the Company’s future insurance needs and costs.
As described above, the Exculpation Amendment would limit the liability of certain officers of the Company in limited circumstances. Other than the addition of a new Article VIII with the text that appears above, and the renumbering of subsequent Articles of the Certificate of Incorporation, the Exculpation Amendment would not cause any changes to the Company’s Certificate of Incorporation. If the Exculpation Amendment is approved by shareholders, the Exculpation Amendment would become effective upon a filing with the Delaware Secretary of State, which the Company would submit promptly following the Annual Meeting of Shareholders.
 | The Board of Directors recommends a vote “FOR” this proposal, for the reasons outlined above. |
 | 2024 Proxy statement | 82 |
To be held at 8:00 a.m. Eastern Time on April 28, 2021.May 6, 2024.
Our Board of Directors is providing these proxy materials to you in connection with a solicitation of proxies for use at the Annual Meeting. You are invited to attend the Annual Meeting and we are asking you to vote on the proposals described in this proxy statement. All stockholdersshareholders as of the close of business on March 1, 2021,7, 2024, will receive the proxy materials and have the ability to access them online at www.proxyvote.com.
Holders of our common stock at the close of business on March 1, 2021,7, 2024, the record date for the Annual Meeting (the “Record Date”)Record Date), are entitled to notice of and to vote at the Annual Meeting. Each stockholdershareholder is entitled to one vote for each share of our common stock held as of the Record Date for each matter addressed at the meeting. As of the Record Date, there were 400,179,947382,879,612 shares of common stock outstanding and entitled to vote. You are entitled to vote shares that are held of record directly in your name and shares held for you as the beneficial owner through a stockbroker,shareholder, bank, or other nominee. For more information, see “Whatisthedifferencebetweenholdingsharesasastockholder shareholder ofrecordandasabeneficialowner?”
The following table shows the proposals to be presented for a vote, the applicable voting requirements, and the Board’s recommendations.
Proposal | Vote required to pass | Voting options | Board’s
| Effect of abstentions and broker non-votes* | |||||
Elect three Class III directors to hold office until the |
| Each director must receive an affirmative vote from a majority of the | FOR, AGAINST or |  |
 |  |
| No effect | |
Approve, on a non-binding, advisory basis, the compensation of our named executive officers |
| A majority of the |  | FOR, |  |
 |
| No effect | |
Ratify the appointment of Ernst & Young LLP as our independent registered public accounting firm for the year ending December 31, |
| A majority of the |  | FOR, |  |
 |
| No effect | |
| Proposal to amend our Certificate of Incorporation to provide shareholders the right to call a special meeting | A majority of shares outstanding | FOR, AGAINST or ABSTAIN |  | FOR | Treated as a vote AGAINST the proposal
| ||||
| Proposal to amend our Certificate of Incorporation to reflect new Delaware law provisions allowing for officer exculpation | A majority of shares outstanding | FOR, AGAINST or ABSTAIN |  | FOR | Treated as a vote AGAINST the proposal | ||||
| * | See “What if I do not specify how my shares are to be voted?” for an explanation of broker non-votes. |
moderna 2021 Proxy statement |58
The Board of Directors knows of no other business to be brought before the Annual Meeting. Should any other matters be presented, the individuals named in the proxy will have the authority to take action in regard to such matters as in their judgment seems advisable. If you hold shares through a broker, bank, or other nominee, they will not be able to vote your shares on any other business that comes before the Annual Meeting unless they receive instructions from you with respect to such matter.
 | 2024 Proxy statement | 83 |
StockholderShareholder of record
If your shares are registered directly in your name with Computershare Trust Company, N.A., our transfer agent, then you are considered the stockholdershareholder of record with respect to those shares. As the stockholdershareholder of record, you have the right to grant your voting proxy directly to the individuals listed on the proxy card or to vote online during the Annual Meeting.
If your shares were held in a stock brokerage account or by a bank or other nominee on your behalf, then you are considered the beneficial owner of shares held in “street name.” As the beneficial owner, you have the right to direct your broker, bank, or other nominee how to vote your shares by following the voting instructions you receive. Your broker, bank, or other nominee has only limited authority to vote your shares without your instructions. For more information, see “What if I do not specify how my shares are to be voted?”ifIdonotspecifyhowmysharesaretobevoted?”
Admission to the Annual Meeting
We will host the Annual Meeting via the Internet. You will not be able to attend the meeting in person. Participation in the Annual Meeting as a stockholder,shareholder, with the right to vote and submit questions, is limited to stockholdersshareholders as of the close of business on the Record Date. The meeting can be accessed at: www.virtualshareholdermeeting.com/MRNA2021.MRNA2024.
Other stockholdersshareholders and members of the public can also access the Annual Meeting at the URL above without a control number, but without the right to vote or submit a question.
The webcast of the Annual Meeting will begin at 8:00 a.m., Eastern Time, on April 28, 2021.May 6, 2024. Online access will begin at 7:45 a.m., Eastern Time, and we encourage you to log into the Annual Meeting 5-15 minutes prior to the start time.
If you encounter difficulties accessing the Annual Meeting, please call the technical support number that will be posted on the registration page for the meeting website.
If you were a stockholdershareholder of record at the close of business on the Record Date, you will need the 16-digit control number found on your Notice of Internet Availability, your proxy card or the instructions that accompany your proxy materials. You do not need to do anything in advance to access the Annual Meeting.
If you were a beneficial owner at the close of business on the Record Date, you may attend the Annual Meeting. You will need the 16-digit control number found on your Notice of Internet Availability, your proxy card or the instructions that accompany your proxy materials if you wish to attend the Annual Meeting with the right to vote and submit a question. Even if you do not have your control number or were not a stockholdershareholder as of the close of business on the Record Date, you can still access the meeting, but will not be able to vote at the meeting or submit a question.
StockholderShareholder of record
If you are a stockholdershareholder of record, you can vote in one of the following ways:
Internet. Go to www.proxyvote.com to complete an electronic proxy card. You will need the control number from the proxy card you receive. You will be responsible for any Internet access charges you incur. If you vote online, you do not need to return a proxy card by mail. Votes by internet must be submitted by 11:59 p.m. on Tuesday, April 27, 2021,Sunday, May 5, 2024, Eastern time, to be counted.
By telephone. telephone.Dial toll-free 1-800-690-6903 and follow the recorded instructions. You will need the control number from your proxy card. You will be responsible for any telephone charges you incur. If you vote by telephone, you do not need to return a proxy card by mail. Votes by telephone must be submitted by 11:59 p.m. on Tuesday, April 27, 2021,Sunday, May 5, 2024, Eastern time, to be counted.
By mail. mail.Complete, date, and sign your proxy card and return it promptly by mail in the envelope provided. The people named in the proxy card will vote your shares in accordance with the instructions you provide. If you return the proxy card, but do not give voting instructions on a particular matter, the people named in the proxy card will vote your shares in accordance with the recommendations of our Board of Directors. Votes by mail must be
moderna 2021 Proxy statement |59
received by the close of business on Tuesday, April 27, 2021,Friday, May 3, 2024, Eastern time, to be counted.
During the meeting. themeeting.If you plan to attend the virtual Annual Meeting, you may vote by following the instructions provided online during the meeting. However, even if you plan to attend the virtual Annual Meeting, we encourage you to submit your vote ahead of time by one of the methods listed above.
If your shares are held in street name, follow the voting instructions you receive from your broker, bank, or other nominee.
 | 2024 Proxy statement | 84 |
StockholdersShareholders as of the Record Date can submit questions in writing in advance of the meeting through www.proxyvote.com. StockholdersShareholders who attend the Annual Meeting and log in as a stockholdershareholder using their control number (as described above) will also have an opportunity to submit questions in writing during the meeting. We will try to answer as many submitted questions as time permits (whether submitted prior to or during the portion of the meeting when questions may be submitted). If we receive substantially similar questions, we will group such questions together and provide a single response to avoid repetition.
StockholderShareholder of record
If you are a stockholdershareholder of record, you may revoke your proxy or change your proxy instructions at any time before your proxy is voted at the Annual Meeting by:
entering a new vote by Internet or telephone;
| • | entering a new vote by Internet or telephone; |
| • | signing and returning a new proxy card with a later date; |
| • | delivering a written revocation to our Corporate Secretary; or |
| • | accessing the Annual Meeting and voting in person. |
signing and returning a new proxy card with a later date;
delivering a written revocation to our Corporate Secretary; or
accessing the Annual Meeting and voting in person.
If you are a beneficial owner, you must contact the broker, bank, or other nominee holding your shares and follow their instructions to change your vote or revoke your proxy.
StockholderShareholder of record
If you submit a proxy but do not provide voting instructions, your shares will be voted:
FOR the election of each of the nominees as Class III directors to serve for a three-year term (Proposal No. 1);
| • | FOR the election of each of the nominees as Class III directors to serve for a three-year term (Proposal No. 1); |
| • | FOR approval, on a non-binding, advisory basis, of the compensation of Moderna’s named executive officers (Proposal No. 2); |
| • | FOR the ratification of the appointment of Ernst & Young LLP as our independent registered public accounting firm for the year ending December 31, 2024 (Proposal No. 3); |
| • | FOR the approval of an amendment to our Certificate of Incorporation to provide shareholders the right to call a special meeting (Proposal No. 4); and |
| • | FOR the approval of an amendment to our Certificate of Incorporation to reflect new Delaware law provisions allowing for officer exculpation (Proposal No. 5). |
| • | In the discretion of the named proxy holders regarding any other matters properly presented for a vote at the Annual Meeting. |
FOR approval, on a non-binding, advisory basis, of the compensation of Moderna’s named executive officers (Proposal No. 2);
FOR the ratification of the appointment of Ernst & Young LLP as our independent registered public accounting firm for the year ending December 31, 2021 (Proposal No. 3); and
In the discretion of the named proxy holders regarding any other matters properly presented for a vote at the Annual Meeting.
If you do not provide voting instructions, then your broker, bank, or other nominee will only have authority to vote your shares with respect to Proposal No. 3 (ratification of the appointment of the independent registered public accounting firm). Your shares will not be voted with respect to Proposals No. 1, 2, 4 and 2.5. This is known as a “broker non-vote.”
A majority of the shares of common stock outstanding and entitled to vote, in person or by proxy, constitutes a quorum for the transaction of business at the Annual Meeting. As of the Record Date, there were a total of 400,179,947382,879,612 shares of common stock outstanding, which means that 200,089,975191,439,807 shares of common stock must be represented in person or by proxy at the Annual Meeting to have a quorum. If there is no quorum, a majority of the shares present at the Annual Meeting may adjourn the meeting to a later date. AbstentionsVotes withheld, abstentions and broker non-votes will be counted for purposes of determining whether we have a quorum.
Proxies are solicited by and on behalf of our Board of Directors. The individuals named in the proxy have been designated as proxy holders by our Board of Directors. When you return a proxy that is properly dated and executed, your shares represented by the proxy will be voted at the Annual Meeting in accordance with your instructions. If you do not give specific instructions on your proxy card, your shares will be voted in
moderna 2021 Proxy statement |60
accordance with the recommendations of our Board of Directors. If any matters not described in this proxy statement are properly presented at the Annual Meeting, the proxy holders will use their own judgment to determine how to vote your shares. If the Annual Meeting is postponed or adjourned, the proxy holders can vote your shares on the new meeting date, unless you have properly revoked your proxy, as described above.
 | 2024 Proxy statement | 85 |
Moderna will bear the entire cost of this proxy solicitation, including the distribution of the proxy materials. Copies of solicitation materials will also be made available to brokers, banks, and other nominees to forward to beneficial owners. The original solicitation of proxies may be supplemented by solicitation by telephone, electronic communication, or other means by our directors, officers, employees, or agents. Morrow Sodali, LLC has been retained to assist in soliciting proxies for a fee of $10,000 plus distribution costs and other expenses.
If you receive more than one proxy card or voting instruction form for the Annual Meeting, your shares may be registered in more than one name or in more than one account. Please follow the voting instructions on each notice you received to ensure that all of your shares are voted.
StockholdersShareholders of Moderna common stock who share a single address may receive only one copy of this proxy statement and Annual Report, unless we have received contrary instructions from any stockholdershareholder at that address. This practice, known as “householding,” is designed to reduce our printing and postage costs and the environmental impact of the Annual Meeting. StockholdersShareholders who participate in householding will continue to receive separate proxy cards if they received a paper copy of proxy materials in the mail. If your household received only a single set of our proxy materials and you would like a separate copy, or if your household received multiple copies of our proxy materials and you want only a single copy next year, please notify our Corporate Secretary at the address provided below. StockholdersShareholders who hold shares in street name may contact their brokerage firm, bank, or other nominee to request information about householding.
Preliminary voting results will be announced at the Annual Meeting. In addition, final voting results will be published in a current report on Form 8-K that we expect to file with the SEC within four business days after the Annual Meeting. If final voting results are not available to us at that time, we intend to file a Form 8-K to publish preliminary results and, within four business days after we know the final results, file an amendment to the Form 8-K to publish those results.
Proposal to include in our proxy statement
StockholdersShareholders may present proper proposals to be included in our proxy statement and considered at the next annual meeting of stockholdersshareholders by submitting their proposals in writing to our Corporate Secretary. For a stockholdershareholder proposal to be considered for inclusion in our proxy statement for our 20222025 annual meeting of stockholders,shareholders (2025 Annual Meeting), our Corporate Secretary must receive the written proposal at our principal executive offices notno later than November 10, 2021,[ ], 2024, unless the date of our 2022 annual meeting of stockholders2025 Annual Meeting is held more than 30 days before or after April 28, 2022,May 6, 2025, in which case the proposal must be received a reasonable time before we begin to print and send proxy materials for the 20222025 Annual Meeting. In addition, stockholdershareholder proposals must comply with the applicable requirements of Rule 14a-8 under the Securities Exchange Act of 1934, as amended.Act.
Our bylaws contain an advance notice procedure for stockholdersshareholders who wish to present a proposal before an annual meeting of stockholdersshareholders but do not intend for the proposal to be included in our proxy statement. These matters may only be brought before the annual meeting by a stockholdershareholder of record entitled to vote at the annual meeting who has delivered timely written notice, containing the information specified in our bylaws, to our Corporate Secretary. To be timely for our 20222025 Annual Meeting, our Corporate Secretary generally must receive the written notice at our
moderna 2021 Proxy statement |61
principal executive offices between December 29, 2021,January 6, 2025, and January 28, 2022.February 5, 2025. However, if we hold our 20222025 Annual Meeting more than 30 days before or more than 60 days after April 28, 2022,May 6, 2025, then notice of a stockholdershareholder proposal that is not intended to be included in our proxy statement must be received no later than the close of business on the later of (a) the 90th day before our 20222025 Annual Meeting and (b) the 10th day following the day on which public announcement of the date of our 20222025 Annual Meeting is first made.
You mayOur Nominating Committee is responsible for selecting and recommending nominees for election as directors to the Board. For information on how you can propose
 | 2024 Proxy statement | 86 |
director candidates for consideration by our Nominating Committee, see “Governance—Composition of Our Board of Directors—Shareholder Recommendations for Director Candidates.”
In addition, a shareholder, or a group of up to 20 shareholders, that has owned continuously for at least three years shares of our common stock representing an aggregate of at least 3% of our outstanding shares, may nominate and Corporate Governance Committee. Forinclude in our proxy materials the greater of two director nominees or the number of nominees constituting 20% of our Board, or if such amount is not a whole number, the closest whole number below 20%, provided that the shareholder(s) and nominee(s) satisfy the requirements in our By-laws. Notice of director nominees pursuant to this proxy access by-law must be received between November [ ], 2024 and December [ ], 2024. However, if we hold our 2025 Annual Meeting more information, see “Governance—Compositionthan 30 days before or more than 60 days after May 6, 2025, then the proxy access notice must be received no earlier than the 120th day before our 2025 Annual Meeting and no later than the close ofOurBoard business on the later ofDirectors.” (a) the 90th day before our 2025 Annual Meeting and (b) the 10th day following the day on which public announcement of the date of our 2025 Annual Meeting is first made.
To directly nominate a director for election at an annual meeting of stockholders, youshareholders, an eligible shareholder must provide the information required by our bylaws.bylaws, including, without limitation, the name and principal occupation or employment of the nominee, the number of shares of our capital stock owned by the nominee, a description of arrangements or understandings between the nominee and the appointing shareholder or shareholders concerning the nominee’s potential service on our Board of Directors and a completed questionnaire with respect to the nominee’s background and qualifications. In addition, youthe appointing shareholder must give notice to our Corporate Secretary in the time frame described above under “--Proposal“—Proposal thatwillnotbeincludedinourproxystatement.”
Furthermore, to comply with universal proxy rules, shareholders who intend to solicit proxies in support of director nominees other than the Company’s nominees must provide notice that sets forth the information required by Rule 14a-19 under the Exchange Act no later than February 5, 2025.
Our bylaws are part of our public filings on the SEC’s website at www.sec.gov. You may also contact our Corporate Secretary at our principal executive officeas described immediately below for a copy of particular bylaw provisions.our bylaws.
You can reach our Corporate Secretary at the following address and phone number: Moderna, Inc., Attention: Corporate Secretary, 200 Technology Square, Cambridge, Massachusetts 02139, (617) 714-6500.714-6500, or via email at Corporate.Secretary@modernatx.com.
We file reports, proxy statements, and other information with the SEC, which is all publicly available on the SEC’s website, http://www.sec.gov. You may also find any document we file with the SEC (and more) on our website at http://www.modernatx.com under the “Investors” heading. Information contained on, or that can be accessed through, our website is not intended to be incorporated by reference into this proxy statement.
You should rely on the information contained in this document to vote your shares at the Annual Meeting. Moderna has not authorized anyone to provide you with information that is different from what is contained in this document. This document is dated March 10, 2021.[ ], 2024. You should not assume that the information contained in this document is accurate as of any later date, and the mailing of this document to stockholdersshareholders at any time after that date does not suggest otherwise. This proxy statement does not constitute a solicitation of a proxy in any jurisdiction where, or to or from any person to whom, it is unlawful to make such proxy solicitations.
moderna 2021 Proxy statement |62
 | 2024 Proxy statement | 87 |
Certificate of Amendment
of
Amended and Restated Certificate of Incorporation
of
Moderna, Inc.
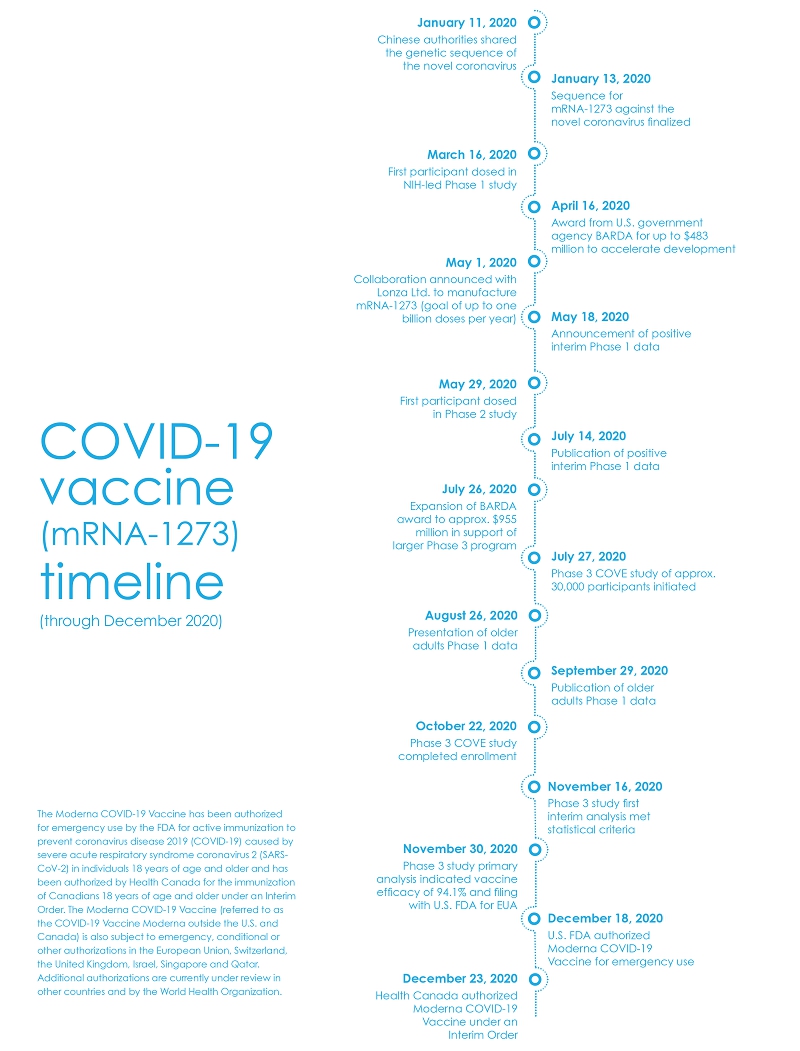


ANNUAL MEETING OF MODERNA, INC.Section 242 of the General Corporation Law of the State of Delaware (the “DGCL”), hereby certifies as follows:
| 1. | ||
|
| |
|
| |
|
|
Please make your marks like this: ☒ Use dark black pencil or pen only
The Board of Directors recommends a vote FOR the nominees listed in Proposal 1 and FOR Proposals 2 and 3.“ARTICLE V”
| 2. | ||||
|
| |||
Nominees:
01 Robert Langer, Sc.D.
02 Elizabeth Nabel, M.D.
03 Elizabeth Tallett
|
|
| ||
| 2. | The Board of Directors of the Corporation has adopted a resolution approving and declaring advisable the foregoing amendment set forth in this Certificate of Amendment in accordance with the provisions of Section 242 of the DGCL. | |
| The stockholders of the Corporation, at a meeting duly called and held pursuant to | ||
| 4. | The foregoing amendments were duly adopted in accordance with Section 242 of the DGCL. |
IN WITNESS WHEREOF, the undersigned, as a duly authorized officer of the Corporation, has executed this Certificate of Amendment on , 2024.
| Moderna, Inc. | ||||||||||||
| Name: Title: |
88
|
Certificate of Amendment
of
Amended and Restated Certificate of Incorporation
of
Moderna, Inc.
Moderna, Inc. (the “Corporation”), a corporation duly organized and existing under the laws of the State of Delaware pursuant to Section 242 of the General Corporation Law of the State of Delaware (the “DGCL”), hereby certifies as follows:
| 1. | The Amended and Restated Certificate of Incorporation of the Corporation, as heretofore amended, is hereby amended by adding a new Article VIII – Limitation of Liability for Officers, which shall read in its entirety as follows: |
“ARTICLE VIII
LIMITATION OF LIABILITY FOR OFFICERS
An Officer (as defined below) of the Corporation shall not be personally liable to the Corporation or its stockholders for monetary damages for breach of his or her fiduciary duty as an officer of the Corporation, except for liability (a) for any breach of the Officer’s duty of loyalty to the Corporation or its stockholders, (b) for acts or omissions not in good faith or which involve intentional misconduct or a knowing violation of law, (c) for any transaction from which the Officer derived an improper personal benefit, or (d) arising from any claim brought by or in the right of the Corporation. If the DGCL is amended after the effective date of this Certificate to authorize corporate action further eliminating or limiting the personal liability of Officers, then the liability of an Officer of the Corporation shall be eliminated or limited to the fullest extent permitted by the DGCL, as so amended. For purposes of this Article VIII, “Officer” shall mean an individual who has been duly appointed as an officer of the Corporation and who, at the time of an act or omission as to which liability is asserted, is deemed to have consented to service by the delivery of process to the registered agent of the Corporation as contemplated by 10 Del. C. § 3114(b).
Any amendment, repeal or modification of this Article VIII by either of (i) the stockholders of the Corporation or (ii) an amendment to the DGCL, shall not adversely affect any right or protection existing at the time of such amendment, repeal or modification with respect to any acts or omissions occurring before such amendment, repeal or modification of a person serving as a Director at the time of such amendment, repeal or modification.”
| 2. | The Amended and Restated Certificate of Incorporation of the Corporation, as heretofore amended, is hereby amended by retitling existing Articles VIII and IX as follows: |
“ARTICLE IX – AMENDMENT OF BY-LAWS”
“ARTICLE X – AMENDMENT OF CERTIFICATE OF INCORPORATION”
| 3. | The Board of Directors of the Corporation has adopted a resolution approving and declaring advisable the foregoing amendment set forth in this Certificate of Amendment in accordance with the provisions of Section 242 of the DGCL. |
| 4. | The stockholders of the Corporation, at a meeting duly called and held pursuant to Section 222 of the DGCL, duly adopted the amendments set forth in this Certificate of Amendment in accordance with the provisions of Section 242 of the DGCL. |
| 5. | The foregoing amendments were duly adopted in accordance with Section 242 of the DGCL. |
IN WITNESS WHEREOF, the undersigned, as a duly authorized officer of the Corporation, has executed this Certificate of Amendment on , 2024.
| ||||||||||||
| Name: Title: | ||||||||||||
89
| ||||||||
 just this portion in the envelope provided. just this portion in the envelope provided. |

Annual Meeting of Stockholders of Moderna, Inc.
to be held on Wednesday April 28, 2021
for Stockholders as of March 1, 2021
This Proxy is being solicited on behalf of the Board of Directors
 |  | |||||||||
| ||||||||||
| ||||||||||
|
| |||||||||
|
|
|
|
|
| ||||||||
| ||||||||
| ||||||||
|
The undersigned hereby appoints Stéphane Bancel, David Meline, and Lori Henderson, and each of them, as the true and lawful attorneys of the undersigned, with full power of substitution and revocation, and authorizes them, and each of them, to vote all the shares of capital stock of Moderna, Inc. which the undersigned is entitled to vote at said meeting and any adjournment or postponement thereof upon the matters specified and upon such other matters as may be properly brought before such meeting or any adjournment or postponement thereof, conferring authority upon such true and lawful attorneys to vote in their discretion on such other matters as may properly come before the meeting and revoking any proxy heretofore given.
THE SHARES REPRESENTED BY THIS PROXY WILL BE VOTED AS DIRECTED OR, IF NO DIRECTION IS GIVEN, SHARES WILL BE VOTED FOR THE ELECTION OF THE DIRECTORS IN PROPOSAL 1, FOR PROPOSALS 2 AND 3 AND IN THE DISCRETION OF THE PROXYHOLDERS ON ANY OTHER MATTER THAT PROPERLY COMES BEFORE THE MEETING.
| ||||||||||||||


 Please separate carefully at the perforation and return just this portion in the envelope provided.
Please separate carefully at the perforation and return just this portion in the envelope provided. 

Proxy for Annual Meeting of Stockholders to be held on Wednesday April 28, 2021
This Proxy is being solicited on behalf of the Board of Directors
Please vote, date and sign this Proxy on the other side and return it in the enclosed envelope.
The Stockholder signing on the reverse side (the “undersigned”), having received the Annual Report and Proxy Statement, hereby appoint(s) Stéphane Bancel, David Meline and Lori Henderson, and each of them, Proxies of the undersigned (with full power of substitution) to attend the Annual Meeting of Moderna, Inc. (the “Company”) to be held on Wednesday April 28, 2021, and all adjournments and postponements thereof (the “Meeting”), and to vote all shares of Common Stock of the Company that the undersigned would be entitled to vote, if personally present, in regard to all matters that may properly come before the Meeting.
The undersigned hereby confer(s) upon the Proxies, and each of them, discretionary authority to consider and act upon such business, matters or proposals as may properly come before the Meeting. The Proxy, when properly executed, will be voted in the manner specified herein. If no specification is made, the Proxies intend to vote FOR all nominees for director in Proposal 1 and FOR Proposals 2 and 3.